Benzoate compound and synthesis method and application thereof
A technology of ester compounds and synthesis methods, which is applied in the field of pollution control of organic small molecule compounds, can solve the problems of inability to reduce the secondary pollution of heavy metal detection reagents and cannot be reused, achieve low synthesis and detection costs, and avoid secondary pollution , the effect of mild synthetic conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
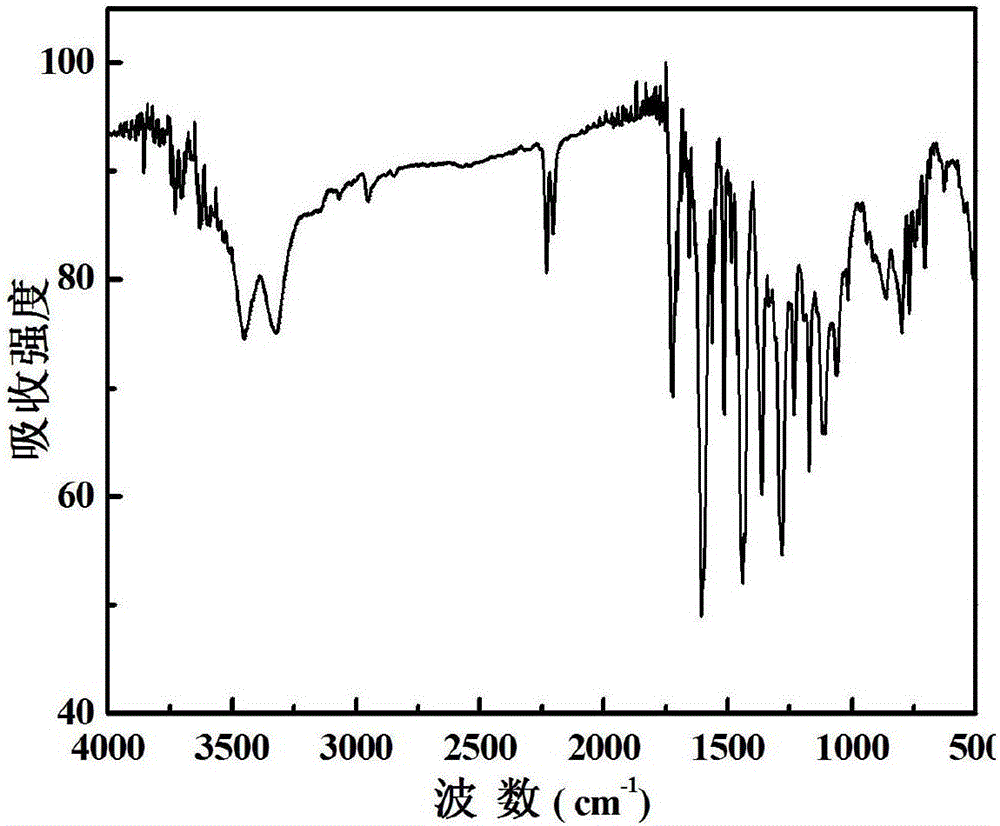
[0050] like figure 1 As shown, the synthetic method of the benzoate compound provided in the embodiment of the present invention comprises:
[0051] S101: add 4-(9-carbazolyl) benzoate to the reactor, add 20 mL to 60 mL of tetrahydrofuran, stir under cooling, add N-bromosuccinimide, and continue stirring overnight after the addition to obtain 4 -[(3-Bromo)-9-carbazolyl]benzoate;
[0052] S102: add 4-[(3-bromo)-9-carbazolyl]benzoate to the reactor, 0.1 g-1 g of anhydrous carbonate, 0.1-1 g of tetrakistriphenylphosphine palladium, 20-1 g of tetrahydrofuran 60mL; N 2 Heating and stirring under protection, adding 5-aldehyde-2-thiopheneboronic acid in the amount of 4-[(3-bromo)-9-carbazolyl]benzoate and other substances dropwise to the reaction solution, and reacting for 3h~ 5h; the product was separated by column chromatography to obtain 4-(3-(5'-formylthiophene)-9-carbazolyl)benzoate;
[0053] S103: add 4-(3-(5'-formylthiophene)-9-carbazolyl)benzoate to the reactor, dissolve ...
Embodiment 1
[0065] Example 1: Synthesis of methyl 4-[(3-bromo)-9-carbazolyl]benzoate
[0066] 4-(9-carbazolyl) benzoic acid methyl ester (2.41g, 4mmol) was added to a 100mL three-necked flask, solvent tetrahydrofuran 30mL, stirred at -30 ° C for 20min, a small amount of N-bromobutane was slowly added for several times Diimide (0.712 g, 4 mmol), stirring was continued for 15 min and at room temperature overnight. Rotary-evaporated, dissolved in chloroform, washed with deionized water (3×100 mL), rotary-evaporated, and recrystallized from absolute ethanol to obtain methyl 4-[(3-bromo)-9-carbazolyl]benzoate. 1 HNMR (400MHz, DMSO, δppm): 8.539 (s, 1H, Ar), 8.333 (d, 1H, Ar), 8.246 (d, 2H, Ar), 7.822 (d, 2H, Ar), 7.584 (d, 2H) ,Ar),7.462(m,3H,Ar),7.391(m,1H,Ar),3.931(s,3H,CH 3 ).
Embodiment 2
[0067] Example 2: Synthesis of .4-[(3-bromo)-9-carbazolyl]benzoic acid ethyl ester
[0068] Same as Example 1, wherein methyl 4-(9-carbazolyl)benzoate is replaced by ethyl 4-(9-carbazolyl)benzoate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com