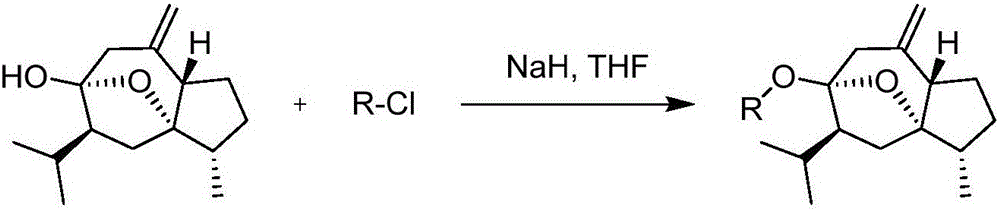Curcumenol derivative as well as preparation method and application thereof in antitumor drugs
A technology of curcumol and derivatives, applied in the field of medicine, can solve the problems of difficult preparation research, low bioavailability, low water solubility, etc., and achieve the effects of improving drug efficacy, good antitumor activity, and increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
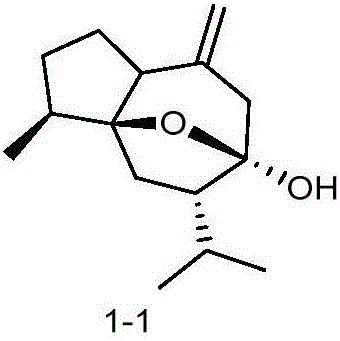
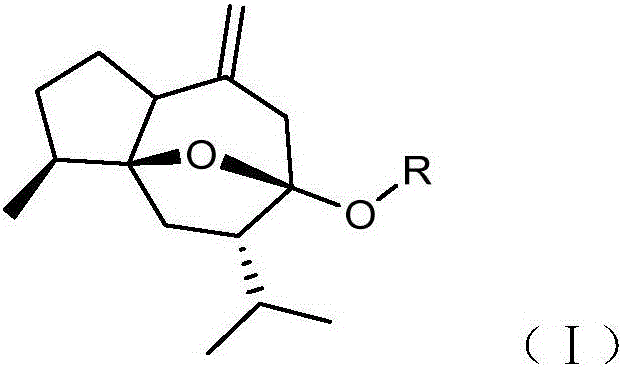
[0027] Embodiment 1: the synthesis of curcumol derivative
[0028]
[0029] The general preparation method takes the synthesis of compound BD-1 as an example:
[0030] Take 1.18g (5mmol) of curcumol, dissolve it in 30mL dry tetrahydrofuran solution, add 0.30g (7.5mmol, 60%) of sodium hydride, heat and reflux at 66-73°C for 1h, let cool slightly, add 2-( Chloromethyl)imidazo[1,2-a]pyridine 1.00g (6mmol), continue to heat up, reflux at 66-73°C for 10h, and monitor the reaction by TLC. After the reaction is complete, remove most of the reaction solvent tetrahydrofuran by rotary evaporation, add 30mL of water, extract 3 times with 40mL of ethyl acetate, combine the ethyl acetate layers, backwash the ethyl acetate layer with saturated sodium chloride aqueous solution, anhydrous magnesium sulfate The ethyl acetate layer was dried appropriately, filtered after 1 h, and concentrated under reduced pressure to obtain a tan oily product, which was further purified by silica gel colum...
Embodiment 2
[0036] In vitro anticancer activity research of embodiment 2 curcumol derivatives:
[0037] MTT method is used to determine the drug concentration when the inhibitory rate of curcumol derivatives to human cervical cancer cell line HeLa cell, human liver cancer cell line HepG2 cell line and human lung cancer cell line A549 cell reaches 50%.
[0038] Tumor cells in the logarithmic growth phase were selected, digested with trypsin, and mixed with RPMI 1640 medium to make 6×10 4 / mL of the cell suspension, and then the cell suspension was added to a 96-well culture plate and cultured for 24 hours at 37°C and 5% CO2. Add the pre-configured drugs with different concentration gradients to the 96-well cell culture plate, set up 3 parallel wells for each concentration gradient, 37°C, 5% CO 2 After culturing for 24 hours under the same conditions, discard the supernatant, wash twice with PBS, add 10 μL of newly prepared MTT medium to each well, continue culturing at 37°C for 4 hours, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com