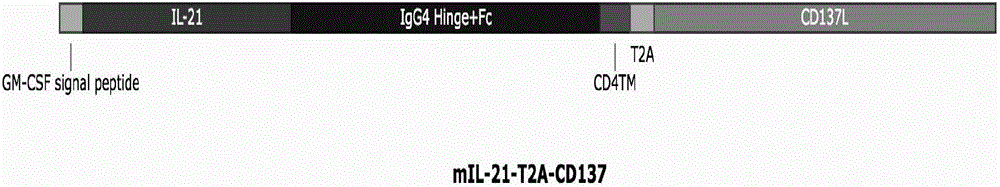
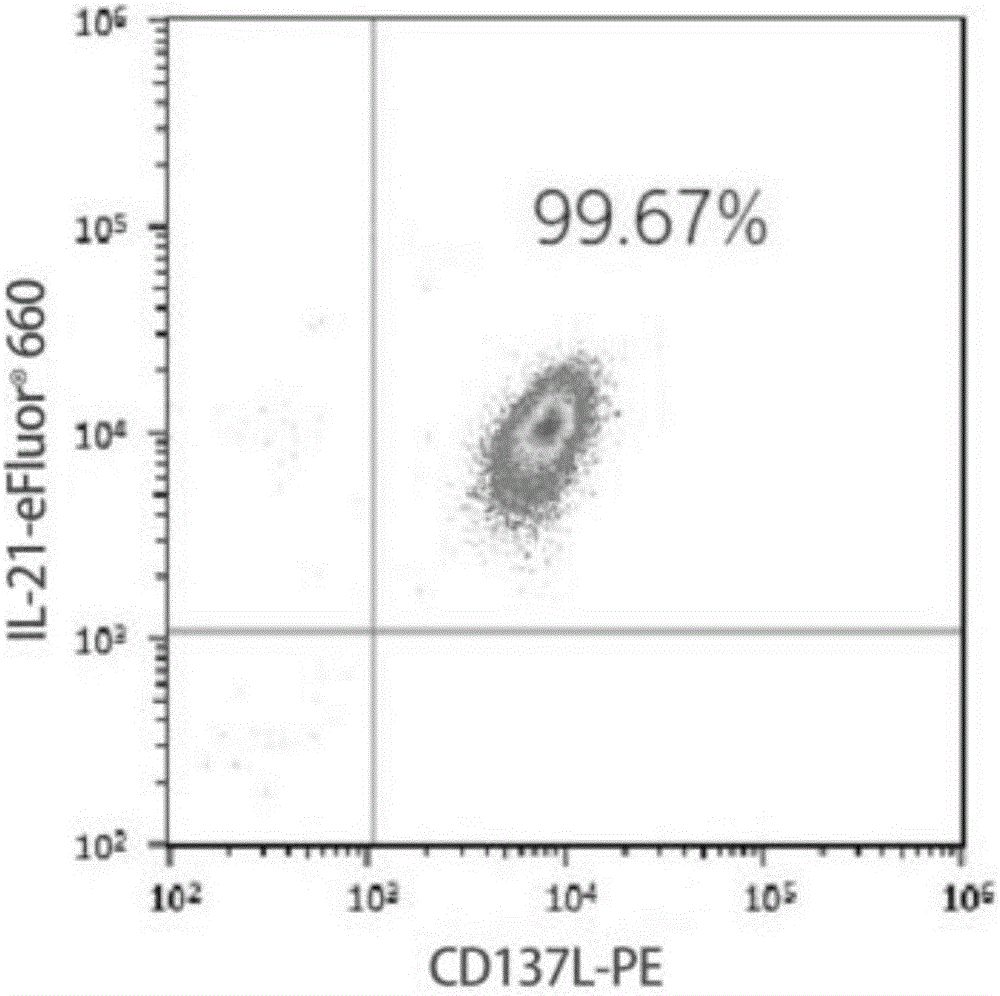
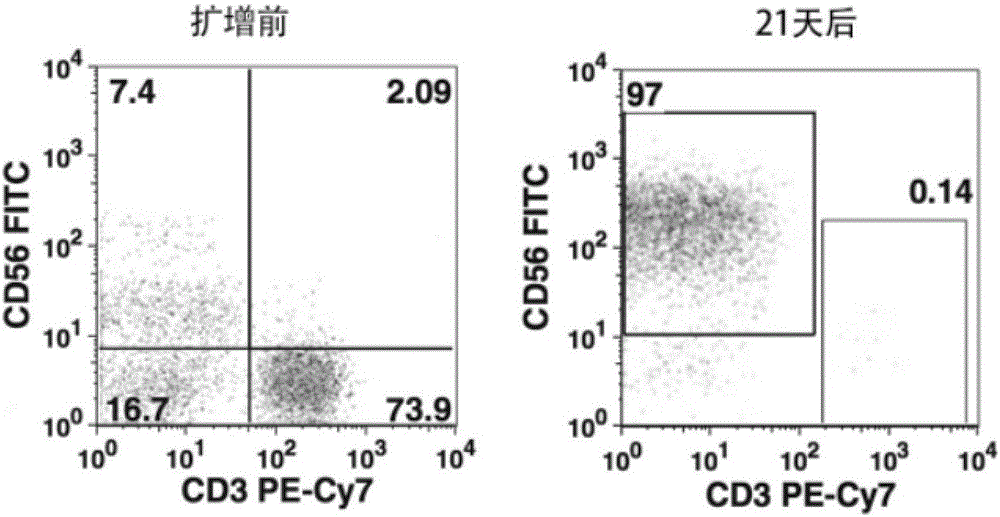
Method for carrying out in-vitro efficient amplification on natural killer (NK) cells, and application of method
A kind of NK cell and high-efficiency technology, which is applied in the field of cell culture and immunotherapy, can solve the problems of cell aging, cytokine dependence, low expansion multiple of NK cells, etc., and achieve life extension, good activity function, purity and cell volume improvement Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] The present invention will be described in further detail below in conjunction with the accompanying drawings.
[0044] Isolation of PBMCs by Density Gradient Centrifugation
[0045] Step1. Take 5 mL of peripheral blood from a 40-year-old middle-aged volunteer and place it in a sterile centrifuge tube, add 0.5 mL of sodium citrate and mix well;
[0046] Step2. Add 5mL1640 cell culture medium to mix again and dilute;
[0047] Step3. Take 5mL of lymphocyte separation solution and place it in a centrifuge tube, slowly add the blood diluted in Step1 to the separation solution along the tube wall with a dropper, and keep the blood and the separation solution separated in two phases;
[0048] Step4. Centrifuge the centrifuge tube at 1800rpm / min for 25min;
[0049] Step5. Insert the mononuclear cell layer along the tube wall with a capillary pipette, wash out the mononuclear cells and place them in another sterile centrifuge tube;
[0050] Step6. Add 5 times the volume of 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com