Oxygen carrier, and preparation method and application thereof
An oxygen carrier and carrier technology, applied in the field of chemical chain hydrogen production, can solve the problems of limited oxygen carrying rate, low cyclic reactivity, high reaction temperature, etc. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 100ml of industrial water glass is treated with 100ml of 1.0M ammonium chloride and 0.5M hydrochloric acid mixture, and the part containing NH is removed by decantation. 4 + and Na + supernatant aqueous solution, the final pH = 10.0, prepared silica-rich sodium silicate hydrated gel (containing SiO 2 60wt%). NaAlO 2 , NaOH, silica gel, cetyltrimethylammonium bromide (CTAB, 98wt%), isomeric cetylamine (CA), tetraethylammonium hydroxide (TEAOH, 35wt%) and water according to Mix in a certain proportion, stir for 4 hours to form a gel, and the molar ratio of each substance in the gel system is Na 2 O:SiO 2 :Al 2 o 3 :CTAB:CA:TEAOH:H 2 O=1:3:0.08:0.8:0.1:0.15:150. The obtained gel was aged at room temperature for 16 hours to obtain a homogeneous gel (pH=11). The gel was heated in a Teflon-lined autoclave for 2 hours at 413K, followed by 100 hours at 443K. The synthesized product was filtered, washed and dried, and calcined at 613K for 3 hours, followed by 823K for...
Embodiment 2
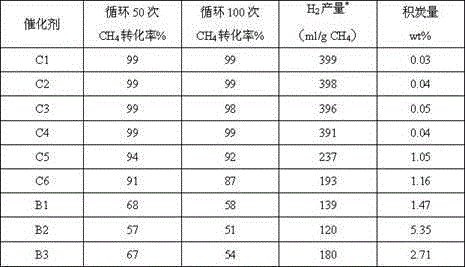
[0021] With embodiment 1, the difference is that the gel system consists of Na 2 O:SiO 2 :Al 2 o 3 :CTAB:CA:TEAOH:H 2O=2:1:0.2:0.4:0.2:0.05:400. Weigh 30g of the carrier, according to the final cerium oxide content of the catalyst of 15%, weigh 25.8g of cerium nitrate and dissolve it in 34ml, add it to the above-mentioned modified carrier for impregnation, and adjust the pH value to 4 with nitric acid at the same time, aging for 2 hours, 80°C Dry for 6 hours and bake at 350°C for 4 hours. Based on the total content of cobalt oxide in the catalyst of 40wt%, 28g of cobalt nitrate was weighed and dissolved in 34ml, added to the above sample, aged for 3 hours, dried at 80°C for 8 hours, and calcined at 350°C for 4 hours. The obtained oxygen carrier is denoted as C-2, and the evaluation results are shown in Table 1.
Embodiment 3
[0023] Same as Example 1, except that the pH value was adjusted to 5 during the preparation of the carrier, and the weight ratio of titanium dioxide and material A in the carrier was 1:10. Weigh 30g of the carrier, according to the final cerium oxide content of the catalyst of 10%, weigh 17.2g of cerium nitrate and dissolve it in 34ml, add it to the above-mentioned modified carrier for impregnation, and adjust the pH value to 5 with nitric acid at the same time, aging for 2 hours, 80°C Dry for 6 hours and bake at 350°C for 4 hours. Based on the total catalyst nickel oxide content of 30wt%, 20.99g of nickel nitrate was weighed and dissolved in 34ml, added to the above sample, aged for 3 hours, dried at 80°C for 8 hours, and calcined at 350°C for 4 hours. The obtained oxygen carrier is denoted as C-3, and the evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com