Preparation method of carbofuran derivatives
A derivative, the technology of carbofuran, which is applied in the field of preparation of carbofuran derivatives, can solve the problems of low solubility, difficulty in recycling, low content of toxic derivatives and low yield, and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
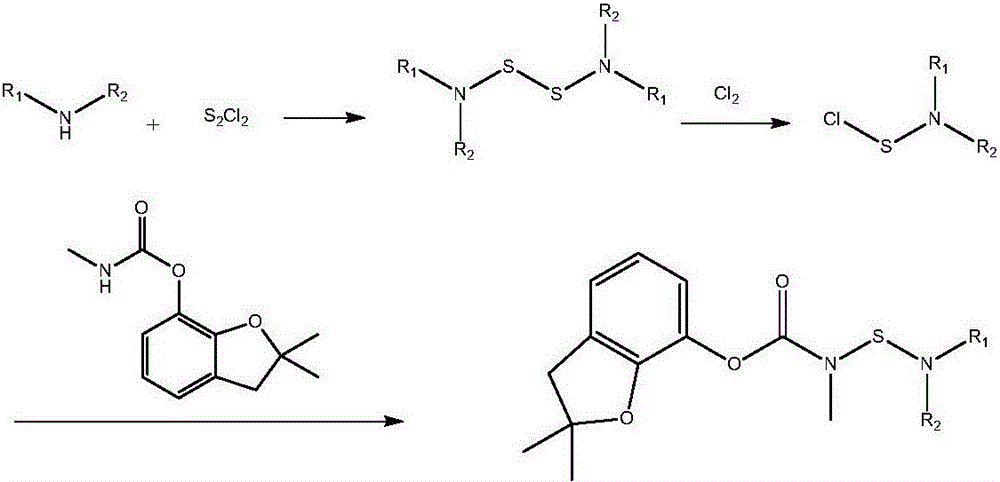
[0030]Carbofuran, chemical name N-[2,3-dihydro-2,2-dimethylbenzofuran-7-yloxycarbonyl(methyl)aminothio]-N-isopropyl-β - Preparation of ethyl alanine
[0031] Add 79.5g (0.5mol, 83mL) N-isopropyl-β-alanine ethyl ester, 0.9L dichloromethane and 50.5g (0.5mol) triethylamine in a stirred 2L reaction flask, cool to 5°C. 33.7 g (0.25 mol) of sulfur monochloride was added dropwise under stirring, and sulfuration reaction was carried out at 5° C. for 1.5 hours after the drop was completed in 1 hour. After the reaction is completed, wash with water, pickle, and wash with water to neutrality to separate the dichloromethane layer (the first intermediate sulfidation reaction product bis-N-isopropyl-β-alanine ethyl ester group disulfide is dissolved in the solvent dichloro in methane). Then the dichloromethane layer was cooled to 5°C, and 17.8g (0.25mol) of chlorine gas was introduced within 1 hour under stirring, and chlorination reaction was carried out at 20°C after the introduction ...
Embodiment 2
[0033] Carbofuran, chemical name N-[2,3-dihydro-2,2-dimethylbenzofuran-7-yloxycarbonyl(methyl)aminothio]-N-isopropyl-β - Preparation of ethyl alanine
[0034] Add 79.5g (0.5mol, 83mL) N-isopropyl-β-alanine ethyl ester, 1.25L dichloromethane and 50.5g (0.5mol) triethylamine in a stirred 2L reaction flask, cool to 5°C. 33.7 g (0.25 mol) of sulfur monochloride was added dropwise under stirring, and sulfuration reaction was carried out at 5° C. for 1.5 hours after the drop was completed in 1 hour. After the reaction was completed, the dichloromethane layer was separated by washing with water, pickling, and washing with water until neutral. Then the dichloromethane layer was cooled to 5°C, and 17.8g (0.25mol) of chlorine gas was introduced within 1 hour under stirring, and chlorination reaction was carried out at 20°C after the introduction was completed, and 105g (0.465mol) was added after reacting for 2 hours. Carbofuran, cool to 5°C, add 101g (1mol) triethylamine dropwise und...
Embodiment 3
[0036] Carbofuran, chemical name N-[2,3-dihydro-2,2-dimethylbenzofuran-7-yloxycarbonyl(methyl)aminothio]-N-isopropyl-β - Preparation of ethyl alanine
[0037] Add 79.5g (0.5mol, 83mL) N-isopropyl-β-alanine ethyl ester, 0.9L chloroform and 50.5g (0.5mol) triethylamine into a stirred 2L reaction flask, cool to 5°C . 33.7 g (0.25 mol) of sulfur monochloride was added dropwise under stirring, and sulfuration reaction was carried out at 5° C. for 1.5 hours after the drop was completed in 1 hour. After completion of the reaction, wash with water, pickle, and wash with water until neutral, and then separate the chloroform layer. Then the chloroform layer was cooled to 5°C, and 17.8g (0.25mol) of chlorine gas was introduced within 1 hour under stirring. After the introduction was completed, the chlorination reaction was carried out at 20°C for 2 hours, and then 105g (0.465mol) of chlorine was added. Cool to 5°C, add 101g (1mol) triethylamine dropwise under stirring, carry out conde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com