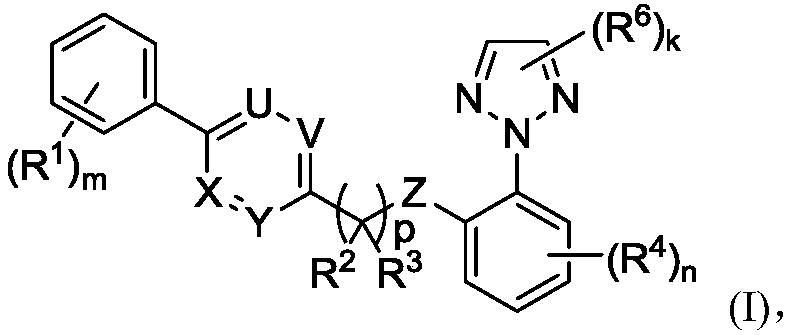
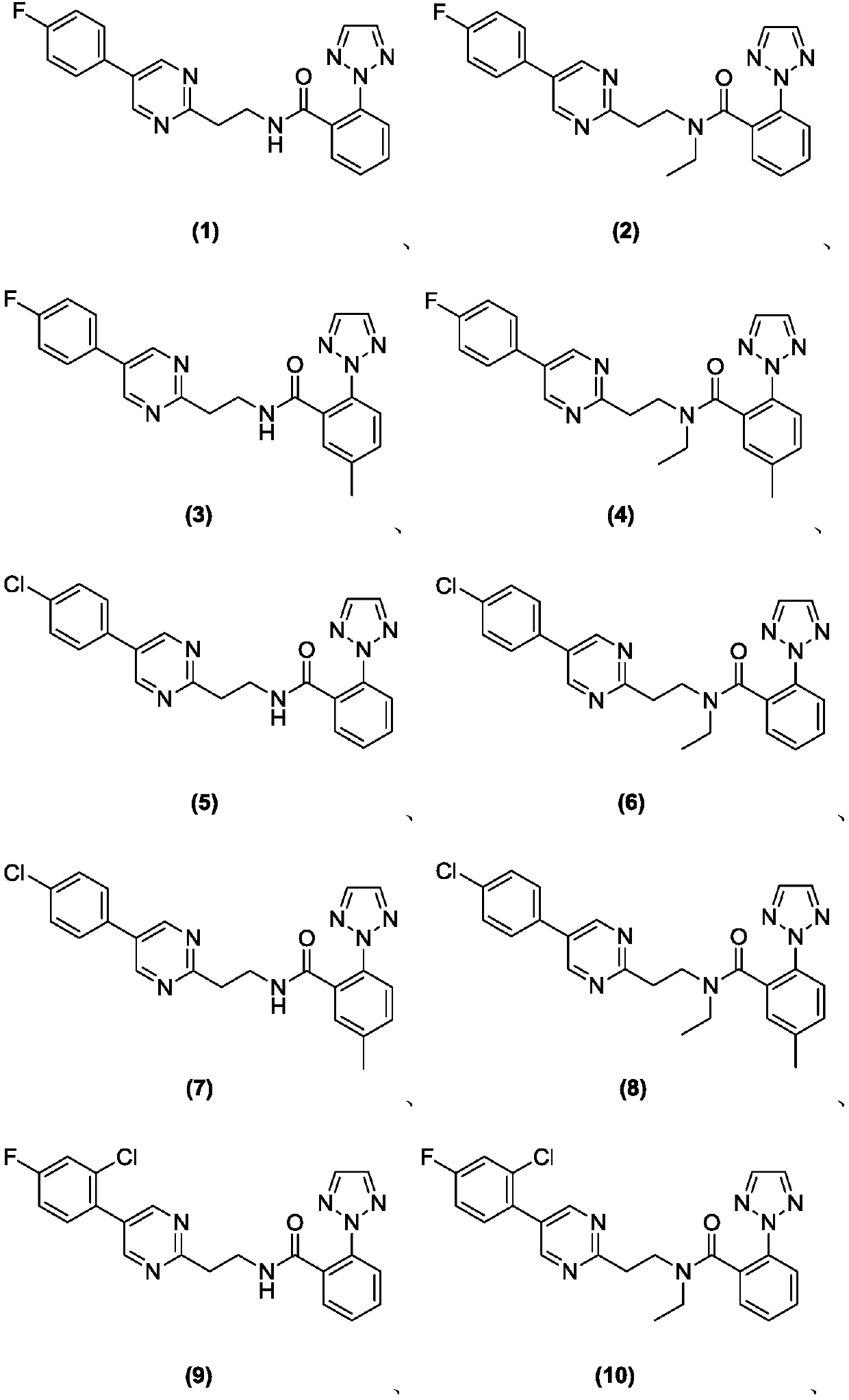
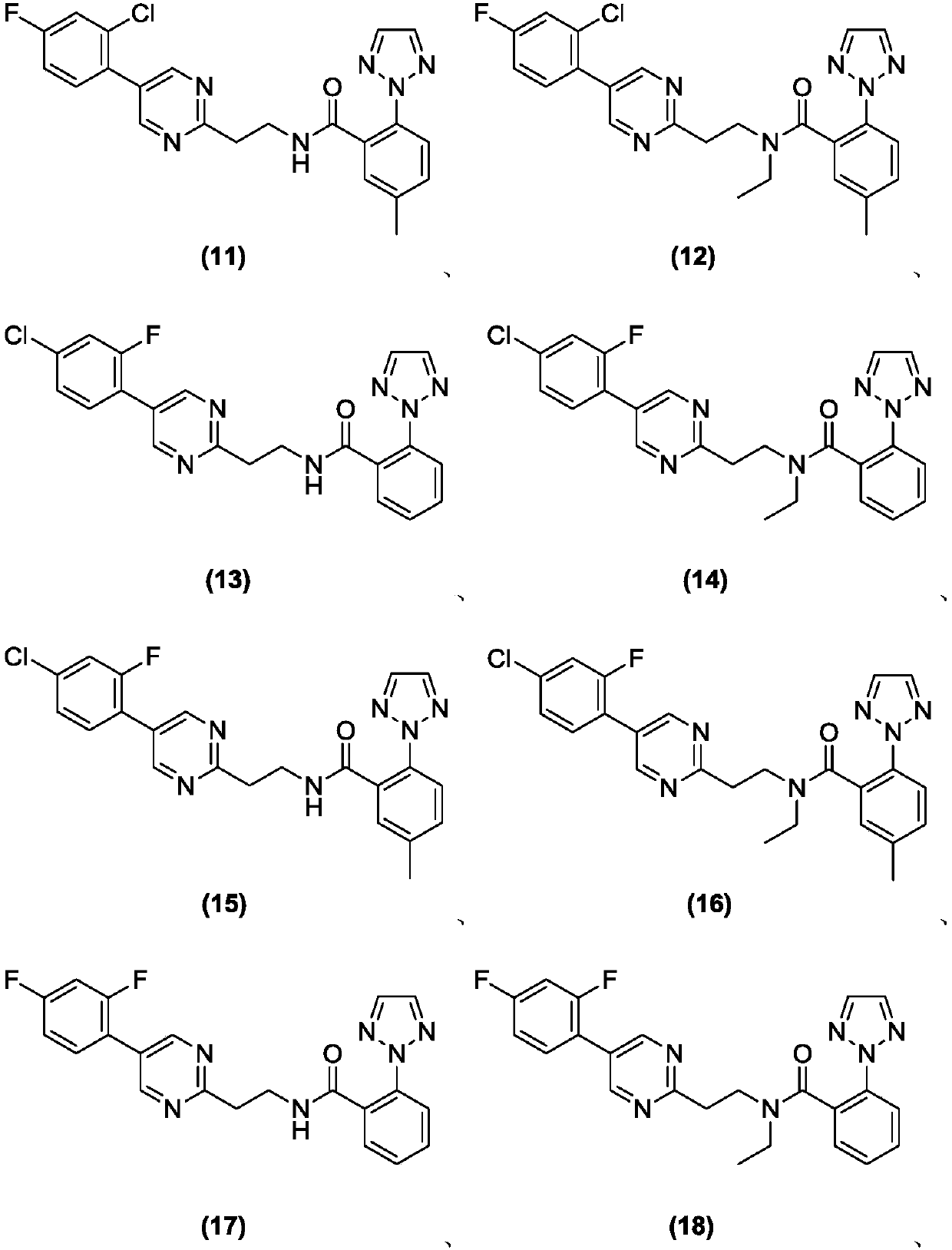
Substituted aryl heteroaryl compound and application thereof
A compound and halogenated alkyl technology, applied in the direction of medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve the problems of safety refusal to approve, and achieve high safety, promote side effects, and good drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Example 1: N-(2-(5-(4-fluorophenyl)pyrimidin-2-yl)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzyl Amide synthesis
[0198]
[0199] Step 1) Synthesis of 2-(5-bromopyrimidin-2-yl)-2-cyanoacetic acid tert-butyl ester
[0200] 5-Bromo-2-chloropyrimidine (5.80g, 30.00mmol), potassium carbonate (12.60g, 90.25mmol) and N-methylpyrrolidone (25mL) were sequentially added to a 100mL reaction flask, followed by slowly adding tert-cyanoacetic acid After the addition of butyl ester (6.50 mL, 45.00 mmol), the reaction temperature was raised to 80° C. for 3 hours. Stop the reaction, pour the reaction solution into water (400mL), adjust the solution to pH=5 with dilute hydrochloric acid, filter with suction, collect the solid, and the crude product is directly separated and purified by silica gel column chromatography (petroleum ether / dichloromethane (v / v) =1 / 5) to obtain the title compound (yellow solid, 3.19 g, 35.72%). MS(ESI,pos.ion)m / z:298.14[M+H] + ;
[0201] 1 H NMR (CDCl 3 ...
Embodiment 2
[0230] Example 2: N-ethyl-N-(2-(5-(4-fluorophenyl)pyrimidin-2-yl)ethyl)-2-(2H-1,2,3-triazole-2 -Synthesis of benzamide
[0231]
[0232] N-(2-(5-(4-fluorophenyl)pyrimidin-2-yl)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide (0.20 g, 0.51mmol) and anhydrous DMF (5mL) were added to a 50mL reaction flask in turn, followed by sodium hydride (0.02g, 0.82mmol), stirred at 0°C for 5 minutes, and bromoethane (0.07g, 0.62mmol ), reacted at room temperature for 2 hours. The reaction was stopped, the reaction solution was diluted with water, extracted with ethyl acetate, the organic phase was collected, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, and the obtained crude product was directly separated and purified by column chromatography (petroleum ether / acetone ( v / v)=5 / 1) to obtain the title compound (pale yellow oil, 0.10 g, 47%).
[0233] MS(ESI,pos.ion)m / z:417.20[M+H] + ;
[0234] HPLC: 95.05%.
Embodiment 3
[0235] Example 3: N-(2-(5-(4-fluorophenyl)pyrimidin-2-yl)ethyl)-5-methyl-2-(2H-1,2,3-triazole-2 -Synthesis of benzamide
[0236]
[0237] Step 1) Synthesis of 5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid
[0238] The title compound of this step was prepared by referring to the method described in step 6 of Example 1, namely 1,2,3-triazole (3.45g, 50mmol), 2-iodo-5-methylbenzoic acid (5.24g, 20mmol) , cesium carbonate (11.72g, 36mmol), trans-N,N'-dimethyl-1,2-cyclohexanediamine (0.51g, 3.6mmol) and cuprous iodide (0.38g, 2mmol) in N , N-dimethylformamide (30mL) was prepared by reacting under nitrogen protection at 100°C for 4 hours, and the crude product was separated and purified by silica gel column chromatography (dichloromethane / methanol (v / v)=50 / 1) to obtain the title compound ( Yellow solid, 2.76 g, 68%).
[0239] MS(ESI,neg.ion)m / z:202.1[M-H] - ;
[0240] 1 H NMR (CD 3 OD,600MHz)δ(ppm):7.88(s,2H),7.66(d,1H),7.59(d,J=8.2Hz,1H),7.50~7.48(dd,J=8.1Hz,1.1Hz,1H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com