Water-soluble cyclic palladium hydrate mono-phosphine salt compound, and preparation method and application thereof
A cyclopalladium hydration and water-soluble technology, applied in the direction of palladium organic compounds, chemical instruments and methods, platinum group organic compounds, etc., to achieve the effects of high yield, mild reaction conditions, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
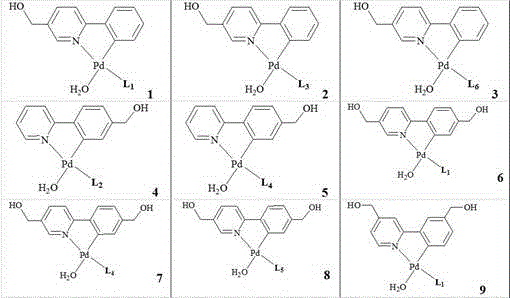
[0025] Water-soluble cyclopalladium hydrated monophosphine internal salt compound, the general formula is:
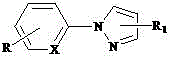
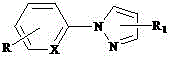
[0026] , wherein L is a monophosphine ligand containing a sulfonic acid group, the benzyl hydroxyl group can be single or on the pyridine ring and the benzene ring at the same time, and the benzyl hydroxyl group can be at any position on the two rings. The specific structure can be:
[0027]
[0028]
[0029] .
Embodiment 2
[0031] Preparation of water-soluble cyclopalladium hydrate monophosphine inner salt compound (1): Add 1 mmol 2-phenyl-5-benzylhydroxypyridine, 1 mmol palladium lithium chloride, 1.2 mmol sodium acetate and 10 ml Anhydrous methanol, after stirring at room temperature for 12 hours, filtered and dried; 1 ) sodium salt was added in acetone solvent, after stirring at room temperature for 3 hours, the filtrate was concentrated with a rotary evaporator, and the raffinate was separated with dichloromethane as a developing solvent with silica gel thin-layer chromatography to obtain water-soluble cyclopalladium hydrated monophosphine inner salt Compound (1), yield 92%. The product (C 42 h 54 NO 5 PPdS) mass spectrometry (ESI) measured data is 821.29.
Embodiment 3
[0033] Preparation of water-soluble cyclopalladium hydrate monophosphine internal salt compound (2): Add 1 mmol 2-phenyl-5-benzylhydroxypyridine, 1 mmol palladium lithium chloride, 1.1 mmol sodium acetate and 10 ml Anhydrous methanol, after stirring at room temperature for 12 hours, filtered and dried; the obtained solid was mixed with 1.1 mmol 3 ) sodium salt was added in acetone solvent, after stirring at room temperature for 3 hours, the filtrate was concentrated with a rotary evaporator, and the raffinate was separated with dichloromethane as a developing solvent with silica gel thin-layer chromatography to obtain water-soluble cyclopalladium hydrated monophosphine inner salt Compound (3), yield 90%. The product (C 30 h 26 NO 5 PPdS) mass spectrometry (ESI) measured data is 649.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com