Light-cured adhesive resin used for dental restoration, and preparation method thereof
A technology of adhesive resin and dental restoration, applied in dental preparations, dentistry, dental prostheses, etc., can solve the problems of lack of bonding performance, poor chemical interface bonding, etc., achieving remarkable improvement effect, simple preparation method, curing Good effect and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
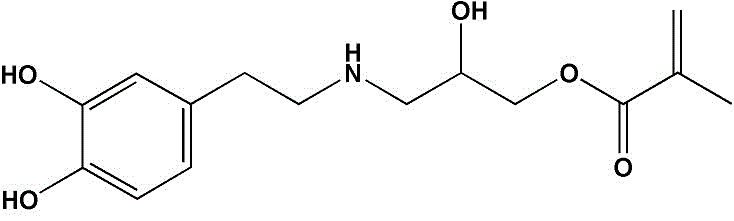
[0028] (a) Add 500ml of anhydrous methanol to a 1L three-neck flask, fill with nitrogen for 30 minutes to remove the oxygen in the system, then add 12g of dopamine hydrochloride, stir until dopamine hydrochloride is completely dissolved, then add 1g of triethylamine catalyst to adjust the pH of the system value of 8.5, then drop 5g of glycidyl methacrylate (GMA) into the system, react at 40°C for 48h, remove methanol by rotary evaporation, and obtain dopamine isohydroxypropyl methacrylate amide (DA-GMA) Crude product; this crude product was dissolved in 500ml of ethyl acetate, washed 3 times with 1M hydrochloric acid solution and saturated brine respectively; separated to obtain the organic layer solution, 3g of anhydrous sodium sulfate was added thereto, and left overnight at room temperature; filtered After removal of insolubles, ethyl acetate was removed by rotary evaporation to obtain purified DA-GMA. The yield of the product was 50%, and its chemical structure was verifie...
Embodiment 2
[0034] (a) The preparation of DA-GMA is the same as step (a) of Example 1.
[0035] (b) Under yellow light conditions, mix 30g Bis-GMA, 30g HEMA and 20g HDDMA, stir well, add 1g CQ and 1g DMAEMA, then stir at 50°C, mix well to get resin matrix A2.
[0036] (c) 0.4 g of DA-GMA was dissolved in 7.6 g of absolute ethanol to obtain a uniform resin matrix B2.
[0037] (d) Mix resin matrix A2 and resin matrix B2, stir evenly, add 10 g of fumed silica, stir mechanically to fully infiltrate and evenly disperse the filler, and obtain adhesive resin mixture C2 for dental restoration. In the binder, the mass percentage of each component is: Bis-GMA (30%), HEMA (30%), HDDMA (20%), DA-GMA (0.4%), CQ (1%), DMAEMA ( 1%), silicon dioxide (10%), ethanol (7.6%).
[0038] The adhesive resin mixture for dental restoration has a double bond conversion rate of 54% in real-time infrared tests; after light curing, the maximum load when the resin is separated from the tooth surface is calculated acc...
Embodiment 3
[0040] (a) The preparation of DA-GMA is the same as step (a) of Example 1.
[0041] (b) Under yellow light conditions, mix 22g Bis-GMA, 25g HEMA and 20g HDDMA, stir well, add 5g CQ and 5g DMAEMA, then stir at 50°C, mix well to get resin matrix A3.
[0042] (c) Dissolve 0.4 g of DA-GMA in 12.6 g of absolute ethanol to obtain a uniform resin matrix B3.
[0043] (d) Mix resin matrix A3 and resin matrix B3, stir evenly, add 10 g of fumed silica, stir mechanically to fully infiltrate and evenly disperse the filler, and obtain adhesive resin mixture C3 for dental restoration. In the binder, the mass percentage of each component is: Bis-GMA (22%), HEMA (25%), HDDMA (20%), DA-GMA (0.4%), CQ (5%), DMAEMA ( 5%), silicon dioxide (10%), ethanol (12.6%).
[0044] The adhesive resin mixture for dental restoration has a double bond conversion rate of 58% in real-time infrared testing; after light curing, the maximum load when the resin is separated from the tooth surface is calculated acco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com