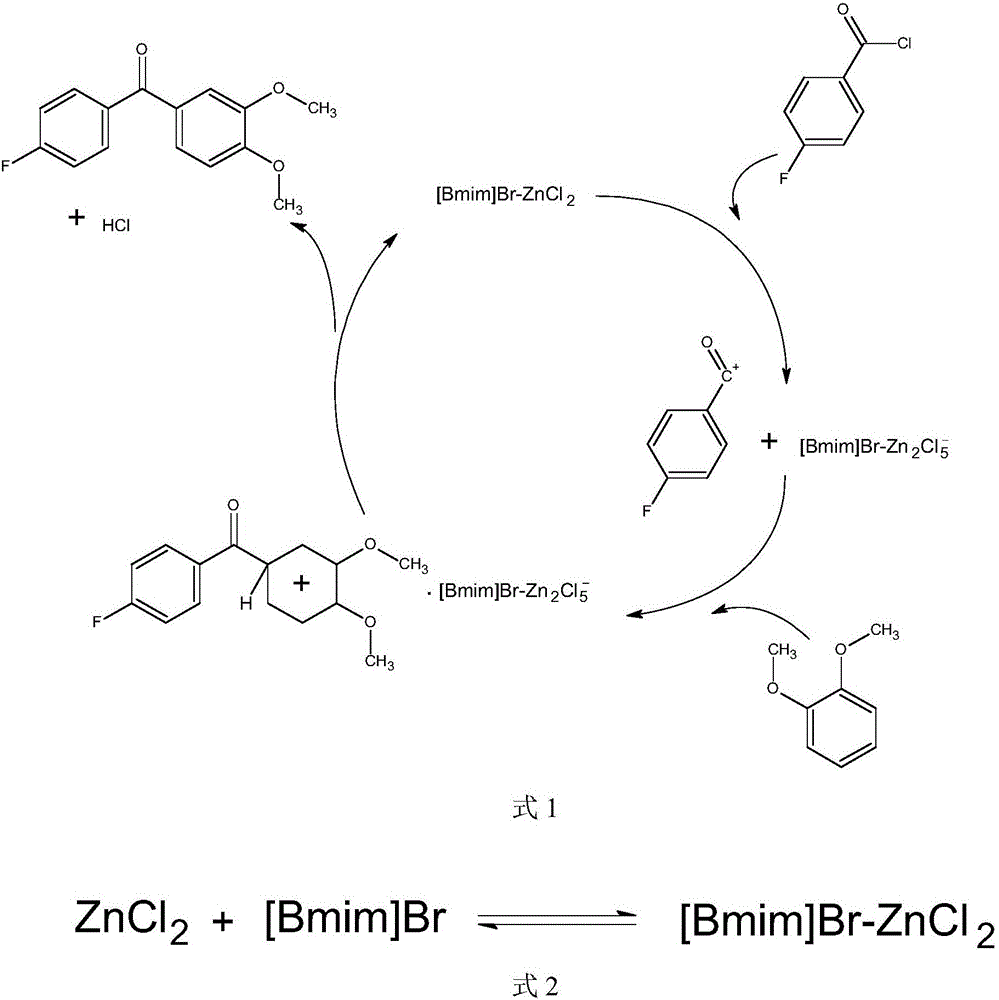
Synthesis method of (3,4-dimethoxyphenyl)(4-p henyl fluoride) ketone
A technology of dimethoxyphenyl and fluorophenyl, applied in the direction of condensation preparation of carbonyl compounds, organic chemistry, etc., can solve the problems of large amount of catalyst usage, discharge of waste water and waste residue, non-recyclability, etc., and achieve less energy consumption, lower The effect of reducing the discharge of waste water and the amount of catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] According to the molar ratio of p-fluorobenzoyl chloride, phthaloyl ether, solvent and catalyst of 1:1:10:0.5, weigh 5 g of p-fluorobenzoyl chloride (chemically pure) and phthalene (chemically pure) respectively. 4.09g, solvent (chemically pure) 40g, ionic liquid catalyst [Bmim]Cl-AlCl 3 8.7g was added into a 100ml three-necked flask, and the solvent was chloroform, the same as in the following examples. The three ports of the flask are respectively equipped with a nitrogen feeding device, a mechanical stirring device, and a condenser. Then, put the flask into an oil bath, turn on the stirring and heating, keep the temperature at 60°C, and the reaction time is 4 hours. After the reaction, wait for it to cool to room temperature, pour the obtained solution into a separatory funnel, add water to the separatory funnel, the amount of water added is twice the volume of the obtained solution, fully hydrolyze the obtained solution, wash, extract, and statically After separati...
Embodiment 2
[0035] According to the molar ratio of p-fluorobenzoyl chloride, phthaloyl ether, solvent and catalyst as 1:1.5:10:0.3, weigh 5 g of p-fluorobenzoyl chloride (chemically pure) and phthalyl ether (chemically pure) 6.13g, solvent (chemically pure) 60g, ionic liquid catalyst [Bmim]Br-ZnCl 2Add 5.22g into a 100ml three-necked flask, install a nitrogen device, a mechanical stirring device, and a condenser at the three ports of the flask, and then put the flask into an oil bath, turn on the stirring and heating, keep the temperature at 60°C, and the reaction time is 4 Hour. After the reaction, wait for it to cool to room temperature, pour the obtained solution into a separatory funnel, add water to the separatory funnel, the amount of water added is 1 times the volume of the obtained solution, fully hydrolyze the obtained solution, wash, extract, and statically After separating the liquid, add water and wash once; the obtained organic phase is subjected to vacuum rotary evaporation...
Embodiment 3
[0037] According to the molar ratio of p-fluorobenzoyl chloride, phthaloyl ether, solvent and catalyst as 1:2:10:0.4, weigh 5 g of p-fluorobenzoyl chloride (chemically pure) and phthalyl ether (chemically pure) 8.17g, solvent (chemically pure) 40g, ionic liquid catalyst [Bmim]Br-CuCl 2 Add 6.96g into a 100ml three-necked flask, install a nitrogen device, a mechanical stirring device, and a condenser at the three ports of the flask, and then put the flask into an oil bath, turn on the stirring and heating, keep the temperature at 60°C, and the reaction time is 4 Hour. After the reaction, wait for it to cool to room temperature, pour the obtained solution into the separatory funnel, add water to the separatory funnel, the amount of water added is 1.5 times the volume of the obtained solution, fully hydrolyze the obtained solution, wash, extract, statically After separating the liquid, add water and wash once; the obtained organic phase is subjected to vacuum rotary evaporation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com