One-step synthetic method of p-(o-)hydroxybenzonitrile
A technology of hydroxybenzonitrile and methyl hydroxybenzoate, which is applied in the direction of organic chemistry, ammonia-carboxylic acid reaction preparation, etc., can solve the problems of increasing equipment investment, large amount of catalyst, and slow reaction process, so as to avoid reaction operation The cumbersome, cost-saving and environmental protection, the effect of improving production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
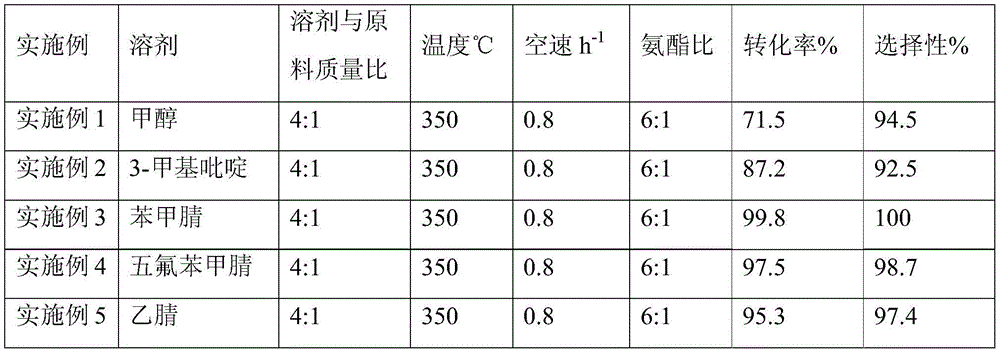
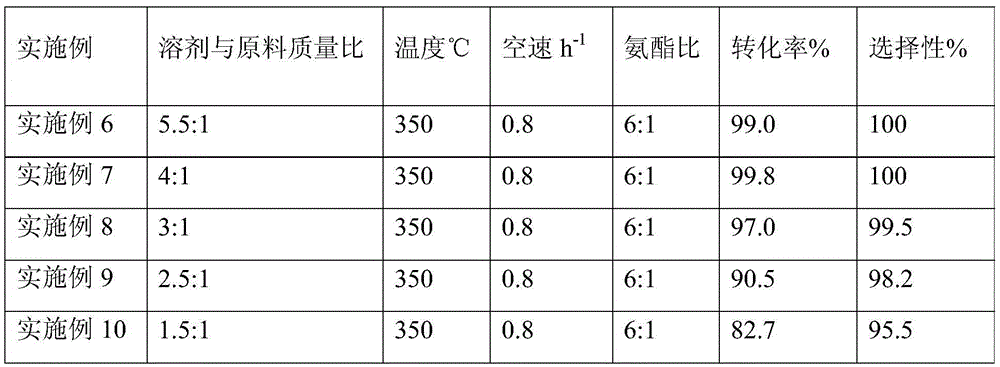
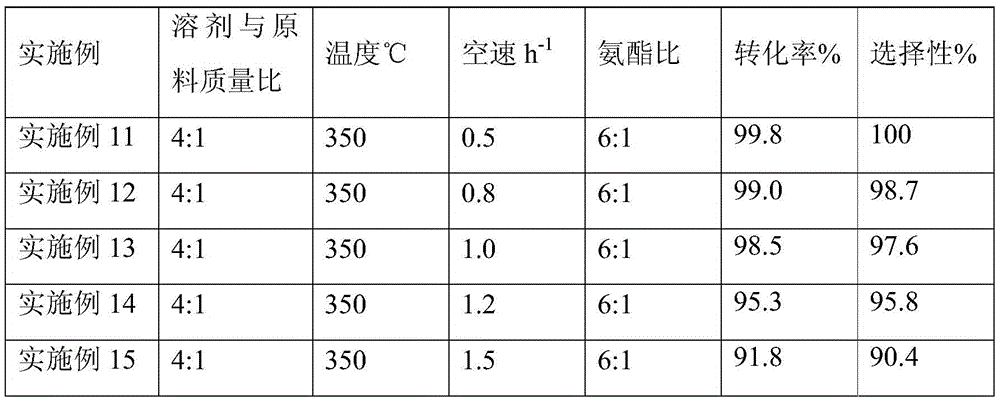
[0021] A kind of one-step synthetic method of p-hydroxybenzonitrile, concrete steps are as follows: methyl p-hydroxybenzoate is dissolved in organic solvent methanol, and methanol and methyl p-hydroxybenzoate mass ratio are 4:1; The methanol solution of methyl formate is measured by a metering pump, and the raw ammonia gas is measured by a gas mass flow meter. The metered methyl p-hydroxybenzoate solution and ammonia gas are mixed through a material mixer and enter into a fixed bed filled with a catalyst through a pipeline. In the reactor, the reaction temperature is 350°C, the reaction pressure is 0.1Mpa (normal pressure); the space velocity of methyl p-hydroxybenzoate is 0.8h -1 , the mass ratio of ammonia to methyl p-hydroxybenzoate is 6:1. The reaction liquid reacted from the fixed bed reactor enters the gas-liquid separation tank after being condensed, and the liquid sample is taken for gas chromatography analysis. The specific results are shown in Table 1.
[0022] Desc...
Embodiment 2
[0029] The organic solvent used in Example 2 is 3-picoline, and others are the same as in Example 1. The specific results are shown in Table 1.
Embodiment 3
[0031] The organic solvent used in embodiment 3 is benzonitrile, and others are the same as embodiment 1, and the specific results are shown in table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com