Catalyst for converting Cr(VI) in aqueous solution and its preparation method and application
A technology of aqueous solution and catalyst, which is applied to the conversion of Cr in water by catalysts, and the synthesis of new catalysts, which can solve the problems of low solar energy utilization rate, achieve the effects of improving visible light absorption, simple process, and improving photocatalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Catalyst Ag / NaTaO3 / Er 3+ :YAlO 3
[0032] (1) Preparation method
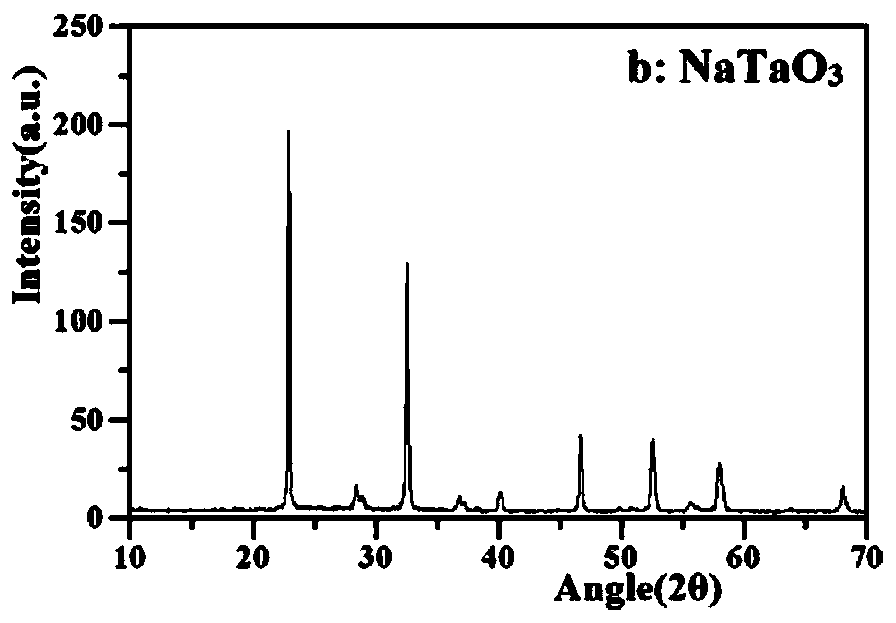
[0033] 1. NaTaO 3 Preparation: 1.7540g Ta 2 o 5 and 3.1750g NaOH (Ta / Na molar ratio 1:10), add 25mL of distilled water and stir evenly, then transfer to a hydrothermal kettle, treat at 180°C for 15h and then cool to room temperature. After the hydrothermal kettle is completely cooled, take it out and discard the supernatant to obtain a white powder, which is centrifuged and washed with deionized water until the eluate is neutral, and then rinsed twice with absolute ethanol. Finally, the product was dried in an oven at 60°C for 12 hours to obtain NaTaO 3 ,spare.
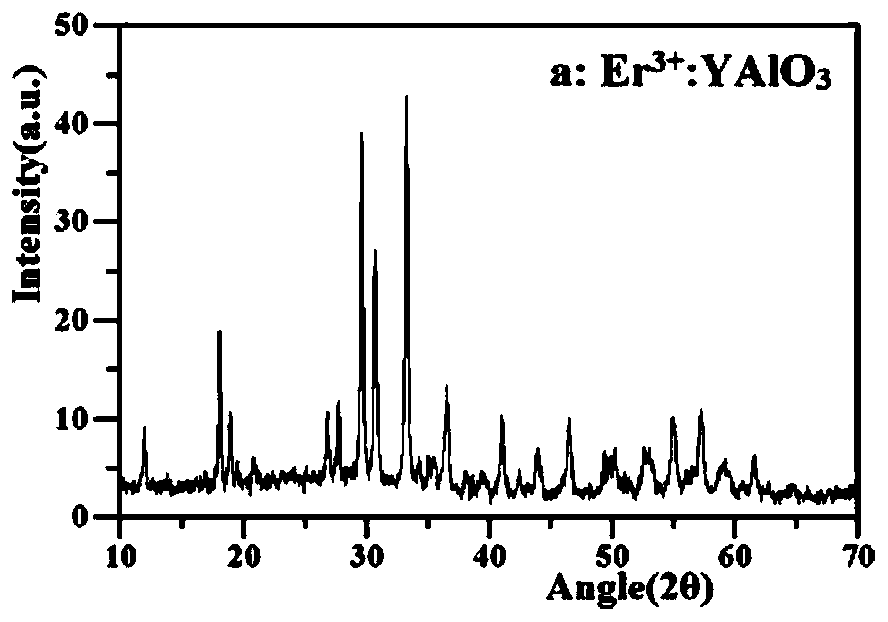
[0034] 2.Er 3+ :YAlO 3 Preparation: 0.0232g Er 2 o 3 , 1.3640g Y 2 o 3 Dissolve in concentrated nitric acid, and magnetically heat and stir until it is colorless and transparent to obtain a rare earth ion solution. Then take another beaker and weigh 4.5316g Al(NO 3 ) 3 9H 2 O was dissolved in distilled water, stirr...
Embodiment 2
[0047] Embodiment 2 Catalyst Au / NaTaO 3 / Er 3+ :YAlO 3
[0048] (1) Preparation method
[0049] 1. NaTaO 3 Preparation: with embodiment 1
[0050] 2.Er 3+ :YAlO 3 Preparation: with embodiment 1
[0051] 3. NaTaO 3 / Er 3+ :YAlO 3 Preparation: with embodiment 1
[0052] 4. Au / NaTaO 3 / Er 3+ :YAlO 3 Preparation: 1g NaTaO 3 / Er 3+ :YAlO 3 Dissolve the powder and 0.01g of chloroauric acid in 200mL of ethanol, and fully disperse for 30 minutes by ultrasonic (80kHZ, ultrasonic output power is 50W) to obtain a suspension, heat the suspension to the boiling point, keep the constant temperature at the boiling point for 30 minutes, filter and washed, the isolated powder was calcined at 350 °C for 1 h and finally ground to obtain Au / NaTaO 3 / Er 3+ :YAlO 3 .
[0053] (2) Characterization data
[0054] Prepared Au / NaTaO 3 / Er 3+ :YAlO 3 XRD and SEM such as Figure 5a with 5b shown. Depend on Figure 4a It can be seen that Au / NaTaO 3 / Er 3+ :YAlO 3 Also has Na...
Embodiment 3
[0058] Example 3Pt / NaTaO 3 / Er 3+ :YAlO 3
[0059] (1) Preparation method
[0060] 1. NaTaO 3 Preparation: with embodiment 1
[0061] 2.Er 3+ :YAlO 3 Preparation: with embodiment 1
[0062] 3. NaTaO 3 / Er 3+ :YAlO 3 Preparation: with embodiment 1
[0063] 4. Pt / NaTaO 3 / Er 3+ :YAlO 3 Preparation: 1g NaTaO 3 / Er 3+ :YAlO 3 Dissolve the powder and 0.01g of chloroplatinic acid in 200mL of ethanol, and fully disperse for 30 minutes by ultrasonic (80kHZ, ultrasonic output power: 50W) to obtain a suspension, heat the suspension to the boiling point, keep the temperature at the boiling point for 30 minutes, filter and washed, the isolated powder was calcined at 350 °C for 1 h and finally ground to obtain Pt / NaTaO 3 / Er 3+ :YAlO 3 .
[0064] (2) Characterization data:
[0065] Prepared Pt / NaTaO 3 / Er 3+ :YAlO 3 XRD and SEM such as Figure 6a with 6b shown. Depend on Figure 6a It can be seen that Pt / NaTaO 3 / Er 3+ :YAlO 3 Also has NaTaO 3 and Er 3+ :Y...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com