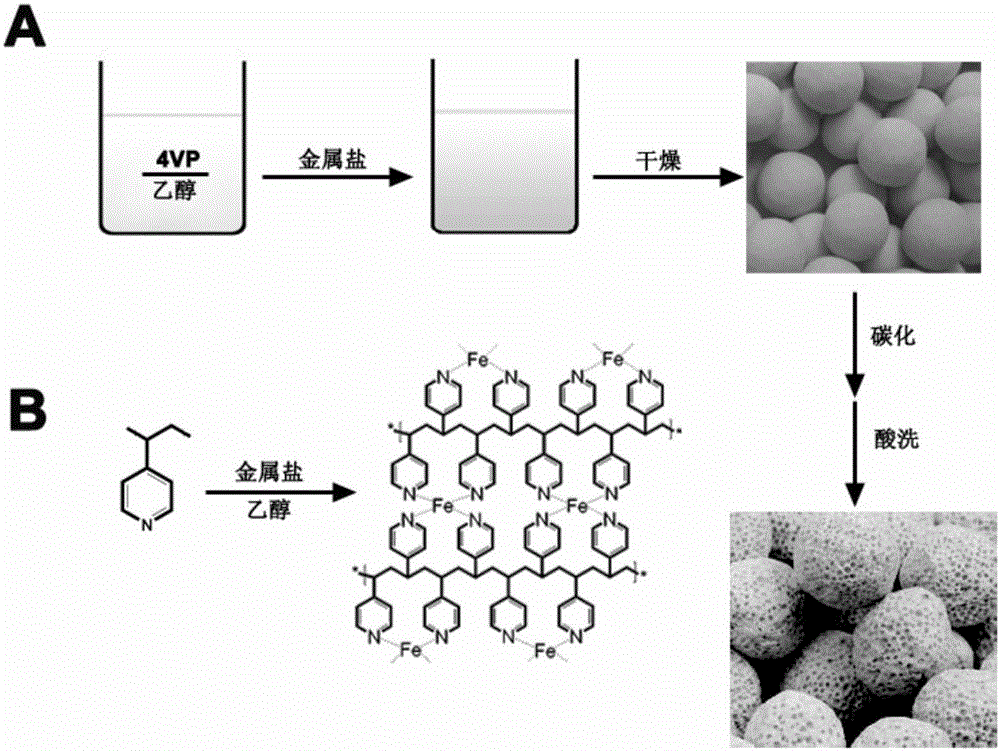
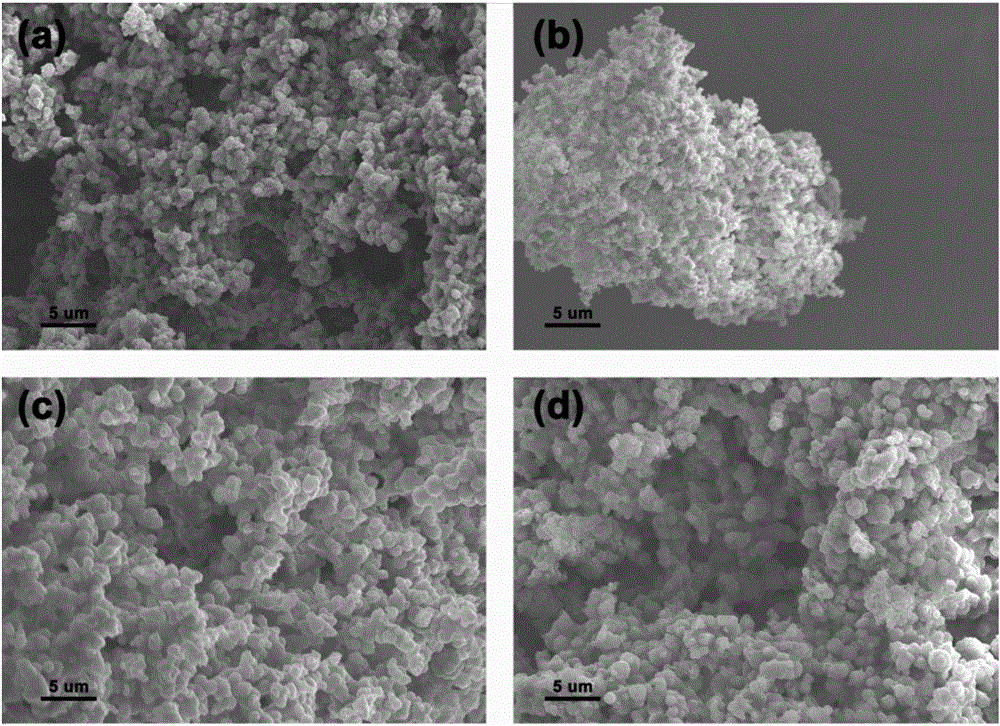
Preparation method of metal-nitrogen-doped porous carbon microspheres
A nitrogen-doped porous carbon and nitrogen-doped carbon technology is applied in the preparation/purification of carbon, which can solve the problems of easy falling off, easy decomposition, poor stability, etc., and achieves large specific surface area, simple steps, and wide selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of 4VP / absolute ethanol solution: at room temperature, take 1.07ml of 4VP monomer and add it to 250ml of absolute ethanol, and magnetically stir to dissolve 4VP to obtain a 4VP / absolute ethanol solution with a concentration of 0.04mol / L for later use .
[0034] (2) Preparation of FeCl 3 / absolute ethanol solution: take 1.62g FeCl 3 Dissolve in 250ml absolute ethanol, magnetically stir to make FeCl 3 Dissolve FeCl with a concentration of 0.04mol / L 3 / absolute ethanol solution for use.
[0035] (3) Fe 3+ - Preparation of organic microspheres: under magnetic stirring conditions at room temperature, take 25ml FeCl 3 / absolute ethanol solution was added to 100ml 4VP / absolute ethanol solution, molar ratio Fe 3+ : 4VP=0.25; react for 12 hours, form a stable complex precipitation, then centrifuge, dry at 60°C for 12 hours to get Fe 3+ -Organic microspheres (Fe 3+ -4VP-0.25°C).
[0036] (4) Carbonization: the Fe 3+ -4VP-0.25 is placed in a quartz bo...
Embodiment 2
[0042] (1) Preparation of 4VP / absolute ethanol solution: at room temperature, take 1.07ml of 4VP monomer and add it to 250ml of absolute ethanol, and magnetically stir to dissolve 4VP to obtain a 4VP / absolute ethanol solution with a concentration of 0.04mol / L for later use .
[0043] (2) Preparation of CuCl 2 / absolute ethanol solution: take 1.34g CuCl 2 Dissolve in 250ml absolute ethanol, magnetically stir to make CuCl 2 Dissolve CuCl with a concentration of 0.04mol / L 2 / absolute ethanol solution for use.
[0044] (3)Cu 2+ - Preparation of organic microspheres: under magnetic stirring conditions at room temperature, take 25ml CuCl 2 / absolute ethanol solution was added to 100ml 4VP / absolute ethanol solution, molar ratio Cu 2+ : 4VP=0.25; after 6 hours of reaction, a stable complex precipitate is formed, and then centrifuged, and vacuum dried at 60°C for 12 hours to obtain Cu 2+ -Organic microspheres (Cu 2+ -4VP-0.25).
[0045] (4) Carbonization: Cu 2+ -4VP-0.25 ...
Embodiment 3
[0049] (1) Preparation of 4VP / absolute ethanol solution: at room temperature, take 1.07ml of 4VP monomer and add it to 250ml of absolute ethanol, and magnetically stir to dissolve 4VP to obtain a 4VP / absolute ethanol solution with a concentration of 0.04mol / L for later use .
[0050] (2) Preparation of MgCl 2 / absolute ethanol solution: take 0.95g MgCl 2 Dissolve in 250ml of absolute ethanol, stir magnetically to make MgCl 2 Dissolved to obtain MgCl with a concentration of 0.04mol / L 2 / absolute ethanol solution for use.
[0051] (3)Mg 2+ - Preparation of organic microspheres: under the condition of magnetic stirring at room temperature, take 50ml MpCl 2 / dehydrated ethanol solution is added to 100ml 4VP / dehydrated ethanol solution, molar ratio Mg 2+ : 4VP=0.5; react for 24 hours, form a stable complex precipitation, and then centrifuge, vacuum dry at 60°C for 12 hours to get Mg 2+ -Organic microspheres (Mg 2+ -4VP-0.5).
[0052] (4) Carbonization: the Mg 2+ -4VP-0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com