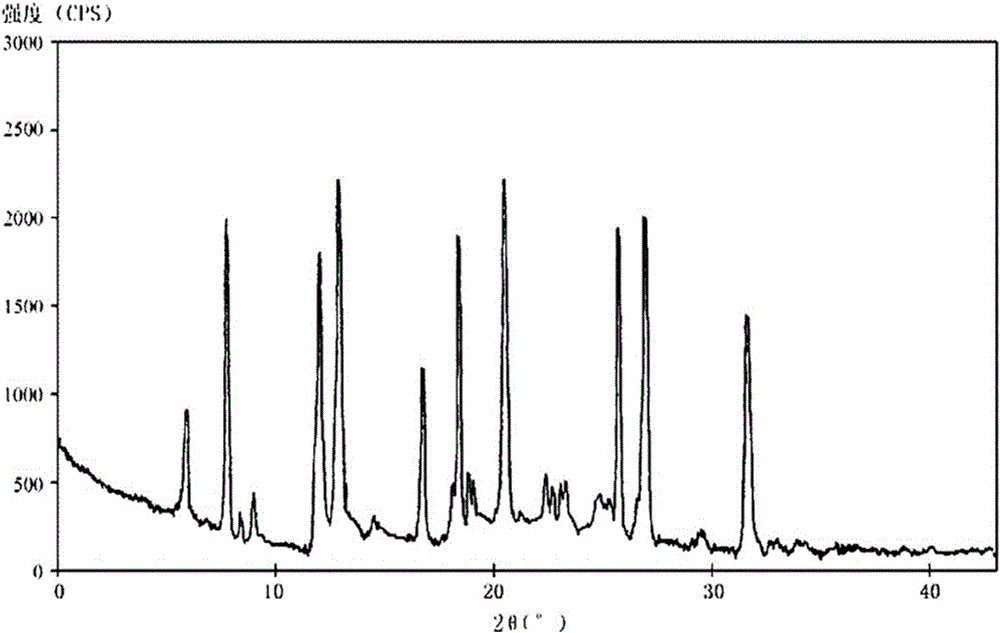
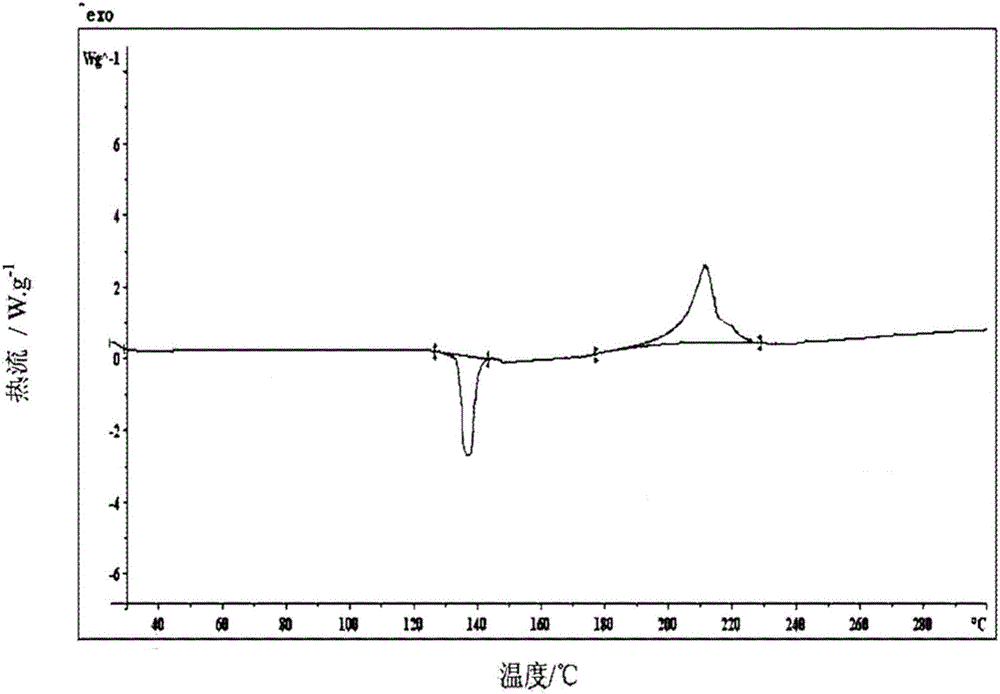
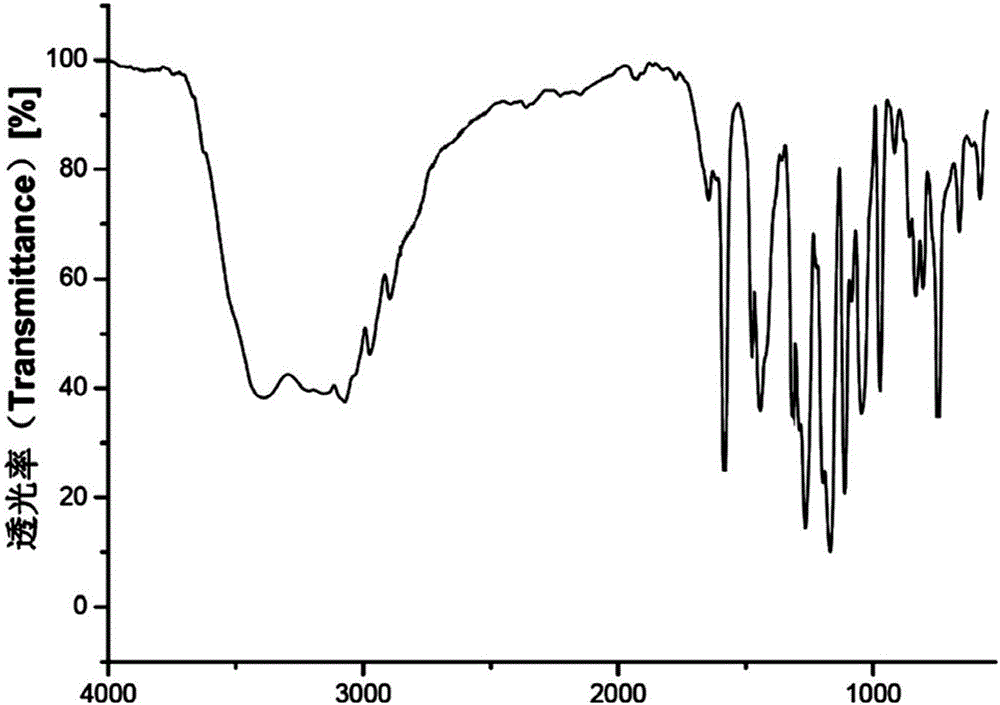
New crystal form of (R)-Lansoprazole sodium and preparation method thereof
A technology of dexlansoprazole sodium and lansoprazole sodium, which is applied in the field of drug preparation, can solve the problems of low stability of preparation products, poor stability of dexlansoprazole, sensitivity to temperature and humidity, etc., so as to avoid sulfoxide The effect of structural oxidation, good crystal morphology, and moderate crystal size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 Preparation of new crystal form A of dexlansoprazole sodium
[0049] Add 50 g of dexlansoprazole sodium into a mixed solvent of 200 mL of dimethylacetamide and 100 mL of purified water, add 0.1 g of copovidone S630, and 0.1 g of propyl gallate, stir and dissolve at 35°C to 40°C, Under the condition of applying ultrasonic wave, the frequency of ultrasonic wave is 20KHz~50KHz, the power is 50W~100W, slowly add 900mL of methyl tert-butyl ether into the above solution, and maintain the cooling rate at 0.3~1℃ / min Under certain conditions, lower the temperature to 0-5°C, continue to stir for 1-3 hours, filter the formed suspension to dryness, and dry it at a temperature of 35-45°C and a vacuum of 0-0.1Mpa to obtain dexlansola The new crystal form A of azole sodium dimethylacetamide solvate is about 57g, and the yield is 93%.
Embodiment 2
[0050] Embodiment 2 Preparation of new crystal form A of dexlansoprazole sodium
[0051] Add 50g of dexlansoprazole sodium into a mixed solvent of 200mL of dimethylacetamide and 200mL of purified water, add 0.1g of PEG4000, and 0.1g of cysteine hydrochloride, stir and dissolve at 35-40°C, and apply Under the condition of ultrasonic action, the frequency of ultrasonic action is 20 KHz ~ 50KHz, the power is 50 W ~ 100W, slowly add 1600mL of methyl isobutyl ether to the above solution, and maintain the cooling rate at 0.3 ~ 1°C / min. , lower the temperature to 0-5°C, continue to stir for 1-3h, filter the formed suspension to dryness, and dry it at a temperature of 35-45°C and a vacuum of 0-0.1Mpa to obtain dexlansoprazole sodium The new crystal form A of dimethylacetamide solvate is about 54g, and the yield is 88%.
Embodiment 3
[0052] Embodiment 3 Preparation of new crystal form A of dexlansoprazole sodium
[0053] Add 50g of dexlansoprazole sodium into a mixed solvent of 200mL of dimethylacetamide and 100mL of purified water, add 0.1g of povidone K30, and 0.1g of butylhydroxyanisole, stir and dissolve at 35-40°C, Under the condition of applying ultrasonic waves, the frequency of ultrasonic waves is 20-50KHz, and the power is 50-100W. Slowly add 900mL of isopropyl ether to the above solution, and keep the cooling rate at 0.3-1°C / min. 0~5℃, continue to stir for 1~3h, suction filter and dry the formed suspension, and dry under the conditions of temperature 35~45℃ and vacuum degree 0~0.1Mpa to obtain dexlansoprazole sodium dimethyl The new crystal form A of acetamide solvate is about 55g, and the yield is 89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com