Antimicrobial peptide based on cell penetrating peptide Tat (49-57) and synthetic method of antimicrobial peptide
A synthesis method and antibacterial peptide technology are applied in the field of antibacterial peptides based on the cell-penetrating peptide Tat and their synthesis, which can solve the problems of unreasonable use, death, multidrug-resistant bacteria and the like, and achieve the effect of good inhibition efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
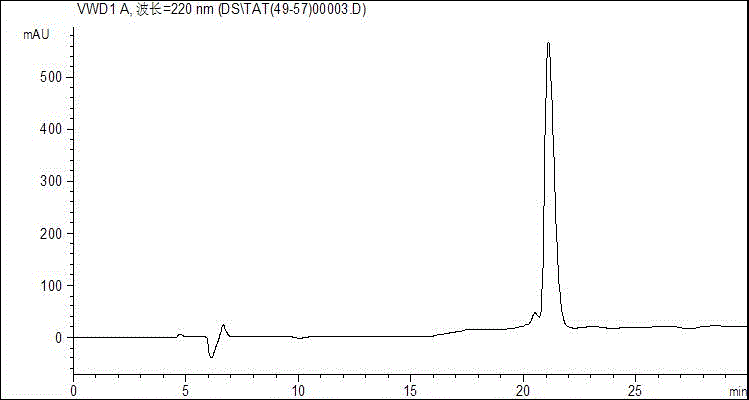
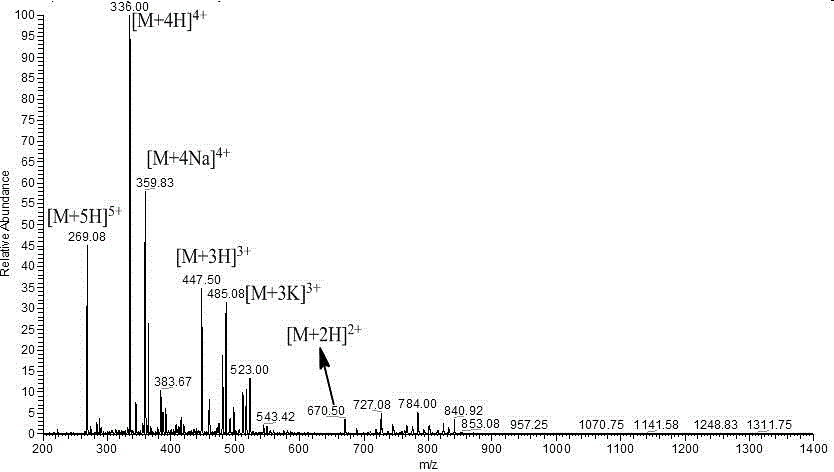
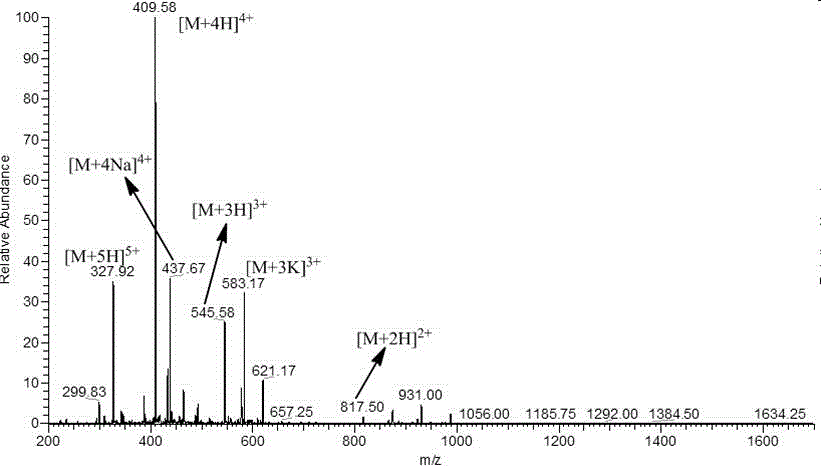
[0025] Embodiment 1--the synthesis of antimicrobial peptide
[0026] 1 Experimental part
[0027] 1.1. Experimental reagents
[0028]
[0029] Preparation of ninhydrin detection solution: Weigh 1 g of ninhydrin and fully dissolve it in 20 mL of absolute ethanol, and the yellow solution formed is the ninhydrin detection solution.
[0030] Preparation of deprotection reagent: Mix 20 mL of piperidine in 80 mL of DMF to make a 20% piperidine DMF solution as the deprotection reagent.
[0031] Preparation of cutting reagent: Mix trifluoroacetic acid, thioanisole, ethanedithiol, water and phenol in a volume ratio of 82.5:5:5:5:2.5, and the mixture is the cutting reagent.
[0032] 1.2 Experimental Instruments
[0033]
[0034] 2. Experimental steps and methods
[0035] 2.1 Solid-phase synthesis of antimicrobial peptides
[0036] The peptide chain sequences of Tat(49-57) and its derived peptides Tat(YG), Tat(YY), Tat(FG), and Tat(FF) are RKKRRQRRR (as shown in SEQ ID No.1), ...
Embodiment 2
[0068] Embodiment 2--antibacterial activity detection of polypeptide
[0069] 1. Experimental Materials and Instruments
[0070] 1.1 Bacteria used in the experiment
[0071] Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Salmonella typhimurium were all commercially available.
[0072] 1.2 Main reagents
[0073]
[0074] 1.3 Main Instruments
[0075]
[0076] 2 Experimental methods
[0077] 2.1 Preparation of main solution
[0078] LB liquid medium: Take 10 g of peptone, 10 g of sodium chloride, and 5 g of yeast powder in a large beaker, add 1000 mL of distilled water, and adjust the pH to 7.2-7.4 with concentrated sodium hydroxide solution after dissolution. The prepared medium was sterilized by moist heat at 121 °C for 20 min, then distributed into 250 mL Erlenmeyer flasks, and stored at room temperature until use.
[0079] MTT test solution: Weigh 100 mg MTT and dissolve it in 20 mL sodium phosphate buffer (pH=7.0), filter through a sterile filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com