Near-infrared and two-photon dual-mode imaging fluorescence probe, as well as preparation and application thereof
A dual-mode imaging and fluorescent probe technology, applied in the field of chemical biology, can solve problems such as unfavorable tumor detection, false positives, and non-targeting Nile red, and achieve good spectral properties and enhanced fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 Preparation of fluorescent probe of the present invention
[0038]Compound 2 (1mmol), compound 3 (1mmol), 1-hydroxybenzotriazole (HOBT, 2.5mmol), N,N-diisopropylethylamine (DIEA, 2.5mmol) and N,N-dimethyl Dimethyl formamide (DMF, 5 mL) was added into a 50 mL one-necked flask, and stirred at room temperature in the dark for 24 hours. Separation and purification with a silica gel column yielded a brown product (67% yield). Detect all-color products, see HRMS spectrum figure 1 , whose structural formula is: , which is the fluorescent probe of the present invention. In this embodiment, the structural formula of compound 2: , the structural formula of compound 3: . The CAS number of compound 3 is 58-85-5. The method of compound 2: 2mmol of 3-diethylaminophenol was slowly added to 5mL of hydrochloric acid with a mass concentration of 18% under the condition of ice bath, and then 2.4 mmol of sodium nitrite was added, and the reaction was more than 12h; a...
Embodiment 2
[0039] Embodiment 2 Fluorescence spectra of fluorescent probes of the present invention in different aqueous solutions
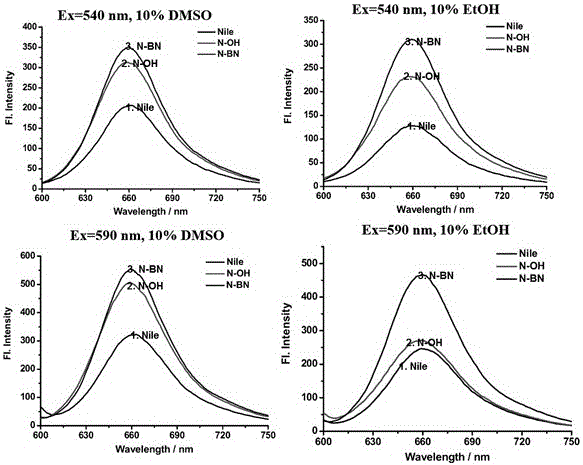
[0040] Using Nile Red, compound 2, and the brown product obtained in Example 1 as fluorescent probes, their fluorescence spectra in different buffers were detected.
[0041] Mix the above-mentioned fluorescent probes with Britton-Robinson buffer solution containing 10% DMSO and Britton-Robinson buffer solution containing 10% EtOH to prepare a buffer solution with a fluorescent probe concentration of 10 μmol / L, and adjust the pH of the buffer solution to Adjusted to 7.54. Then take 540nm and 590nm as the excitation wavelength (λ ex =540nm, 590nm) for fluorescence detection; the obtained fluorescence spectrum is as follows figure 2 shown. according to figure 2 It can be drawn that the fluorescence spectrum of the probe obtained with Nile Red, compound 2, and the brown product obtained in Example 1 has a maximum emission wavelength at 660 nm, and the peak...
Embodiment 3
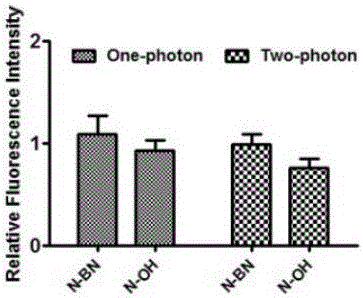
[0042] Embodiment 3 The fluorescence intensity of the fluorescent probe of the present invention in positive and negative cells
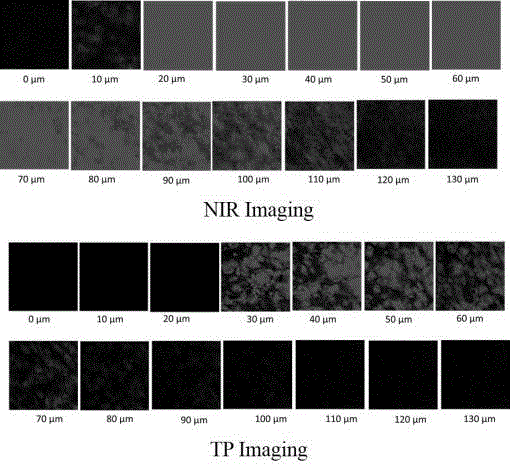
[0043] Compound 2 (N-OH for short) and the brown product obtained in Example 1 (N-BN for short) were used as fluorescent probes to detect their fluorescence spectra in positive and negative cells. The positive cells are cell lines with high biotin receptor expression (HeLa); the negative cells are cells with low biotin receptor expression (NIH3T3).
[0044] Cells HeLa (positive cells) with high expression of biotin receptors or NIH3T3 cells (negative cells) with low expression of biotin receptors were inoculated into 35 mm confocal small dishes and cultured for 24 hours, and then replaced with 5 μmol / L fluorescent probe) Cell culture medium was incubated for 15 minutes, washed three times with PBS, and placed under a confocal microscope (equipped with a femtosecond laser) to detect the fluorescence imaging results of positive or negative cells in fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com