Vero cell line for stably expressing bovine trypsinogen (S.pro-try) and purpose thereof
A bovine trypsin and cell line technology, applied to cells modified by introducing foreign genetic material, medical preparations containing active ingredients, enzymes, etc., can solve the difficulty of increasing cell culture, accelerate multi-cycle growth of viruses, and improve Vaccine production costs and other issues, to achieve the effect of simplifying the virus culture process, increasing the virus yield, and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Construction and Identification of the Bovine Pancreatin Gene Eukaryotic Expression Vector of Recombination Optimization in Example 1
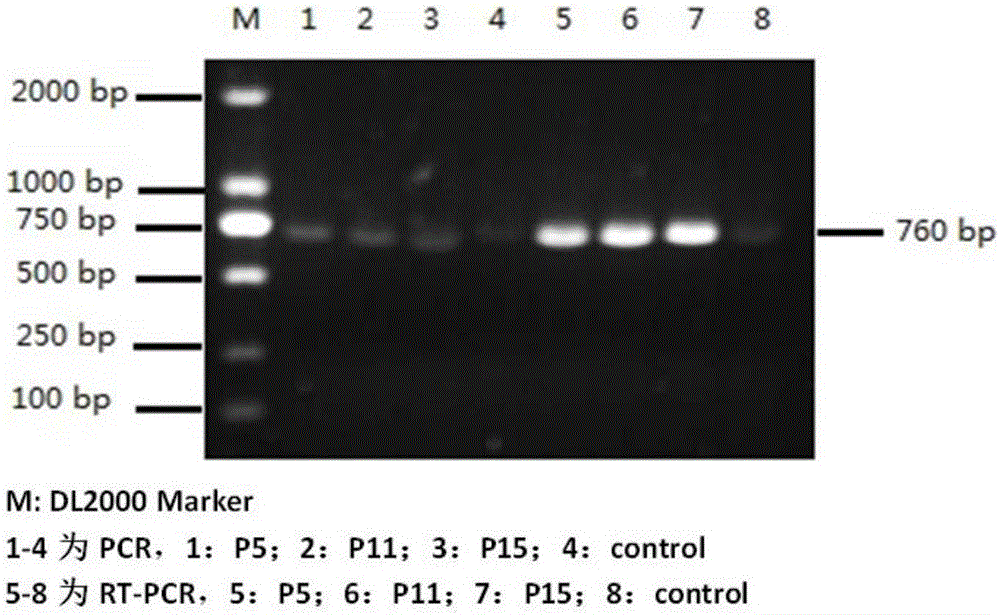
[0041] (1) Vector construction: using the bovine trypsinogen cDNA sequence provided by GenBank (Genbank LOC780933, namely SEQ ID No.1) as a template, design specific primers, upstream primer CCC GCCACCATGAAGACCTTCATCTTTC (SEQ ID No.2), downstream primer CCG TTAGTTGGAGGACATGGTCTGC (SEQ ID No.3), amplified to obtain the expression sequence of the secreted zymogen, and simultaneously introduced a single restriction site in the upstream and downstream respectively, the upstream primer was introduced into EcoR I (shown in italic underline), and the downstream primer was introduced into Xho I ( Italic underlined), adding the Kozak sequence GCCACC to the N-terminus of the initiation codon ATG of the upstream primer to improve the expression efficiency. In a 50 μl PCR reaction system, 5 μl of amplification template, 32.8 μl of sterilized dou...
Embodiment 2
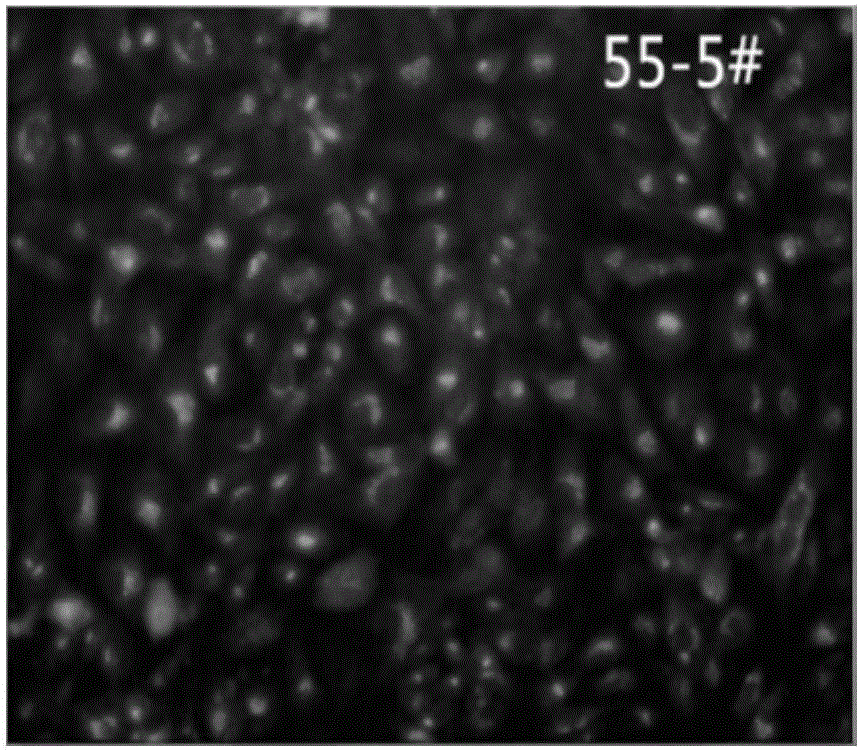
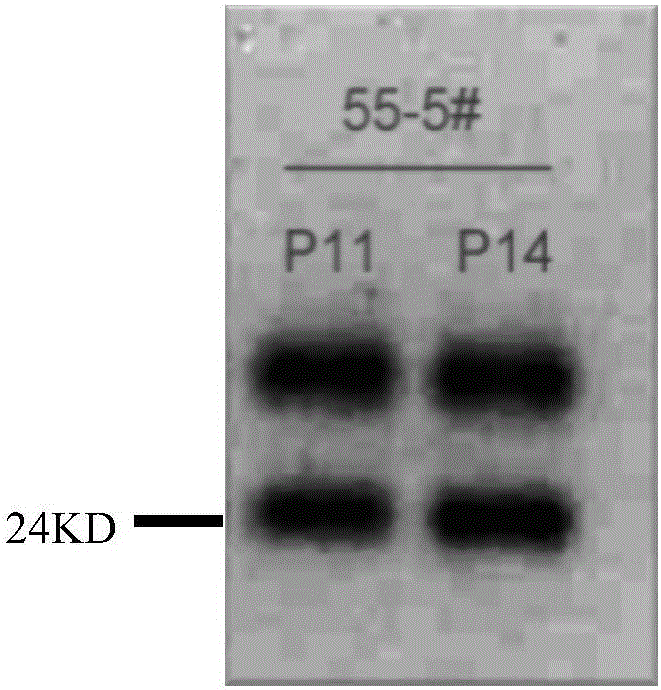
[0044] Example 2 Screening and identification of monoclonal cells stably expressing secretory trypsinogen Vero
[0045] (1) Selection of the appropriate G418 concentration in the screening medium: inoculate Vero cells into a 24-well plate and add G418 when the confluence is 70%, so that the concentration is 0 μg / ml, 200 μg / ml, 400 μg / ml, 600 μg / ml , 800μg / ml, 900μg / ml, 1000μg / ml, 1100μg / ml, duplicate wells for each concentration, placed at 37°C, 5% CO 2 Cultivate in an incubator, observe and record cell death every day, change the medium every 3-4 days, draw a cell survival curve after two weeks of cultivation to determine the minimum lethal concentration of G418, and determine the concentration of G418 in the Vero cell pressure screening medium in the experiment. 600μg / ml, the maintenance solution concentration is 300μg / ml.
[0046] (2) G418 pressurized selection and limiting dilution method for screening monoclonal cells: inoculate Vero cells into a 6-well plate, culture un...
Embodiment 3
[0048] Example 3 Proliferation of influenza virus on Vero cells stably expressing bovine trypsinogen
[0049]Monoclonal cells VTY55 and normal Vero were inoculated in 6-well cell culture plates to 90% confluence, aspirated off the cell culture medium, washed cells with sterile PBS 3 times to remove FBS, replaced with serum-free DMEM containing 1% double antibody and incubated at 37°C Cultivate in the box for 24 hours, and wait for the expression and accumulation of the zymogen of the monoclonal cells. After 24 hours, each subtype of influenza virus was inoculated, and the trypsin group was added with a final concentration of 0.5 μg / ml TPCK-Trypsin (TPCK-Trypsin) at the same time. After mixing, they were cultured in a 37°C 4% CO2 incubator. Freeze and thaw the cells twice at 48h, 72h, and 96h respectively to release the virus, centrifuge at 5000rpm at 4°C for 10min to remove cell debris, collect the supernatant virus liquid, and detect the virus NA activity. Results: The monoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com