Poly(fluorenyl ether nitrile) crosslinked anion exchange membrane material with functionalized cationic groups for fuel cell and preparation method of the poly(fluorenyl ether nitrile) crosslinked anion exchange membrane material
A technology of anion exchange membranes and cationic groups, applied in fuel cells, electrochemical generators, circuits, etc., can solve the problems of poor alkali resistance stability and low anion conductivity of anion exchange membranes, and achieve excellent alkali resistance stability, Good alkali resistance and stability, good for storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
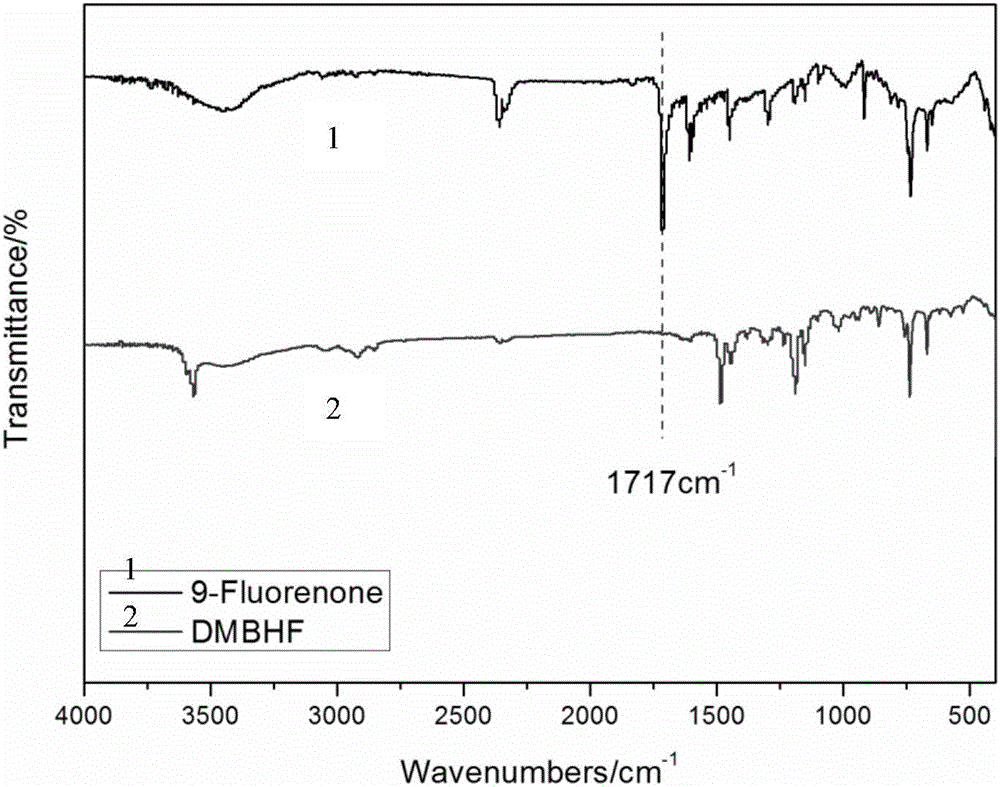
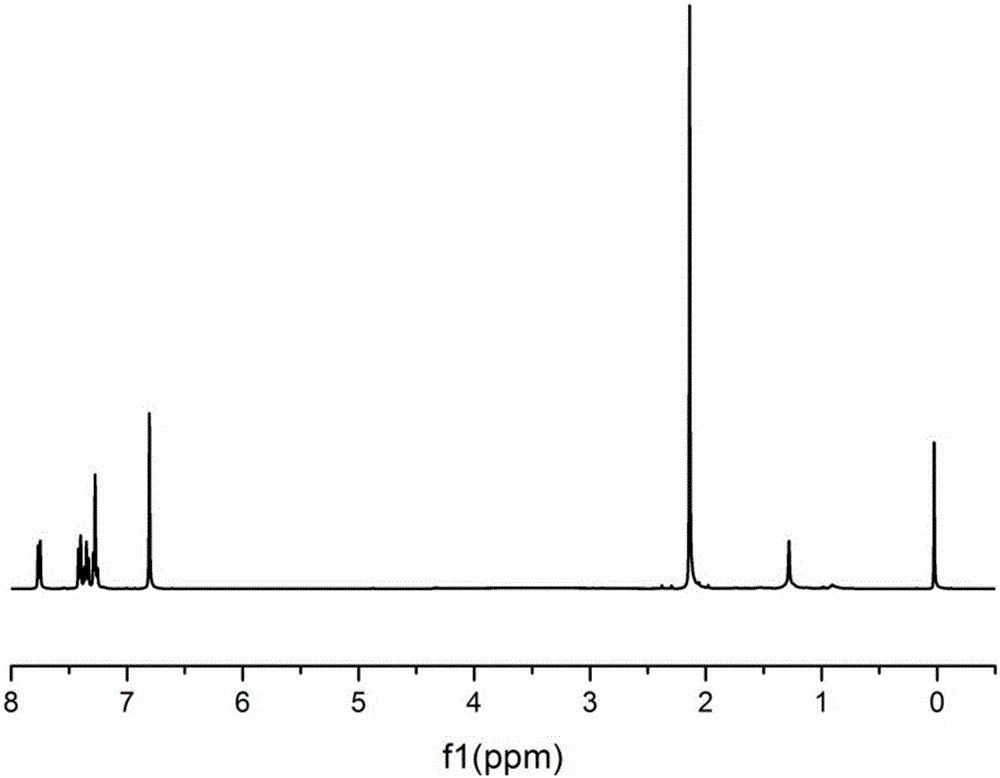
[0038] Under nitrogen protection, add 0.04mol 9-fluorenone, 0.12mol 2,6-dimethylphenol, 0.1mL 3-mercaptopropionic acid, 10mL toluene to a 100mL three-necked flask, and add 1.5mL at 30°C with a mass fraction of 98% Concentrated sulfuric acid at 55°C for 5h, and discharged in distilled water. Suction filtration obtained a light yellow solid crude product, which weighed 15.9 g after drying. Add the crude product and 200mL of toluene into a 500mL three-necked flask, stir at 90°C for 3h, and filter under reduced pressure while hot to obtain the filtrate. After cooling and standing still, the white crystalline product is obtained by suction filtration, and dried to obtain 9,9-bis(3,5- Methyl-4-hydroxyphenyl)fluorene (DMHPF), weighing 9.1g.
Embodiment 2
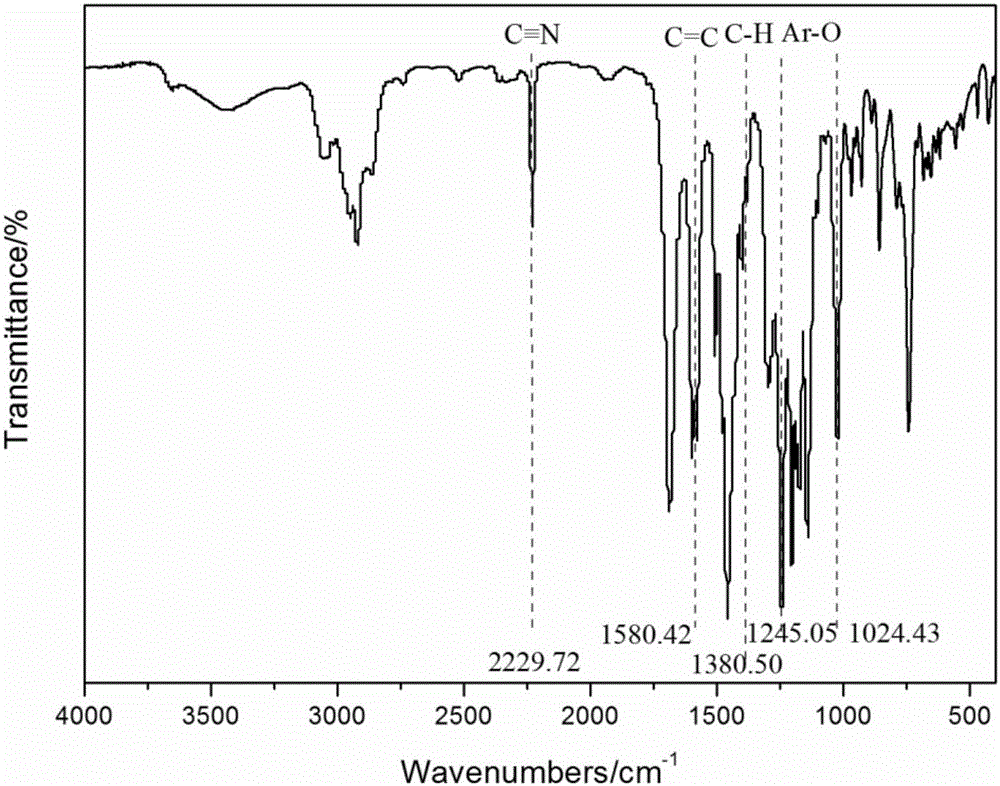
[0040] Under nitrogen protection, add 0.006mol (2.439g) 9,9-bis(3,5-methyl-4-hydroxyphenyl ) fluorene (prepared according to the method of Example 1), 0.009mol (3.026g) hexafluorobisphenol A, 0.015mol (2.087g) 2,6-difluorobenzonitrile, 20mL sulfolane, 0.01875mol (2.5875g) potassium carbonate, 15mL of toluene, reflux at 128°C, carry water for 4 hours, let go of the water-carrying agent; raise the temperature to 180°C, distill the toluene, continue the reaction for 10 hours, and discharge the material in water to obtain a strip-shaped polymer. The polymer was crushed with a pounder, boiled 6 times with distilled water, filtered, and dried in a vacuum oven at 40°C for 48 hours to obtain 7.1 g of benzylmethyl-containing polyfluorene ether nitrile polymer. The benzylmethyl group accounted for 160 mole percent of the resulting polymer.
Embodiment 3
[0042] Under nitrogen protection, add 0.009mol (3.659g) 9,9-bis(3,5-methyl-4-hydroxyphenyl ) fluorene (prepared according to the method of Example 1), 0.006mol (2.017g) hexafluorobisphenol A, 0.015mol (2.087g) 2,6-difluorobenzonitrile, 20mL sulfolane, 0.01875mol (2.5875g) potassium carbonate, 15mL of toluene, reflux at 128°C, carry water for 4 hours, let go of the water-carrying agent; raise the temperature to 180°C, distill the toluene, continue the reaction for 10 hours, and discharge the material in water to obtain a strip-shaped polymer. The polymer was crushed with a masher, boiled 6 times with distilled water, and dried in a vacuum oven at 40°C for 48 hours; 7.2 g of benzylmethyl-containing polyfluorene ether nitrile copolymer was obtained. The benzylmethyl group accounted for 240 mole percent of the resulting polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Methanol permeability coefficient | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com