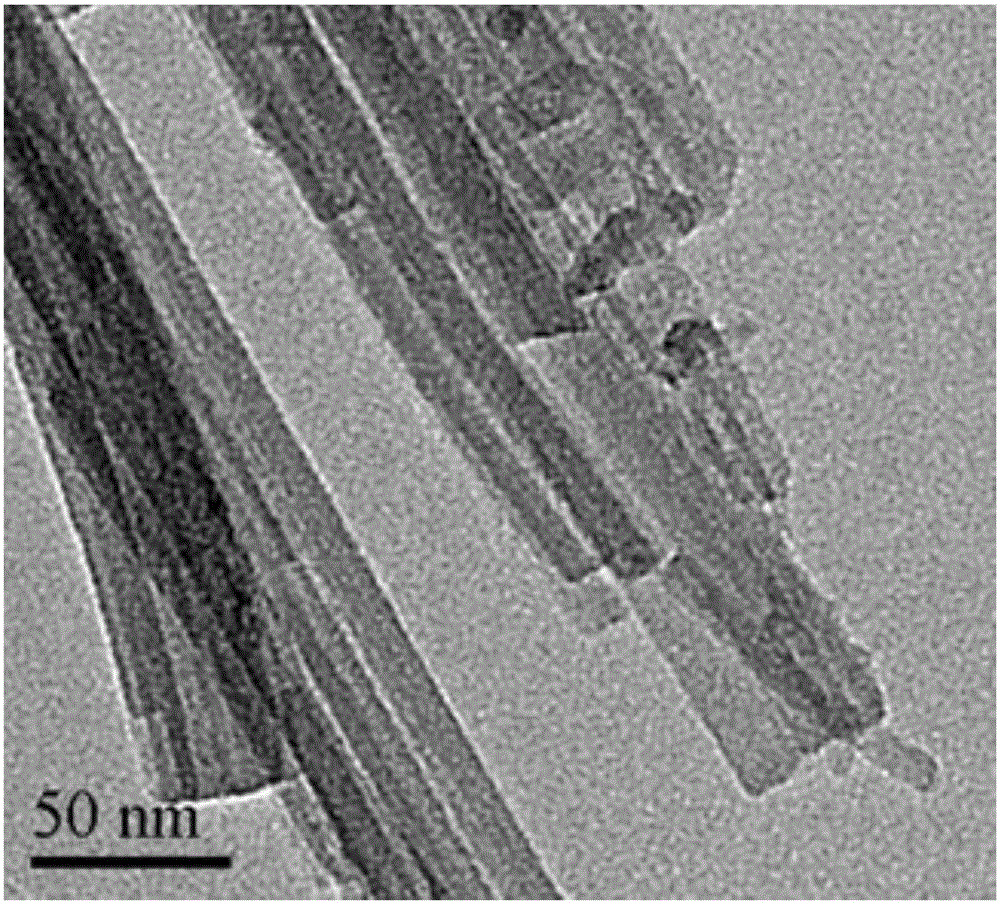
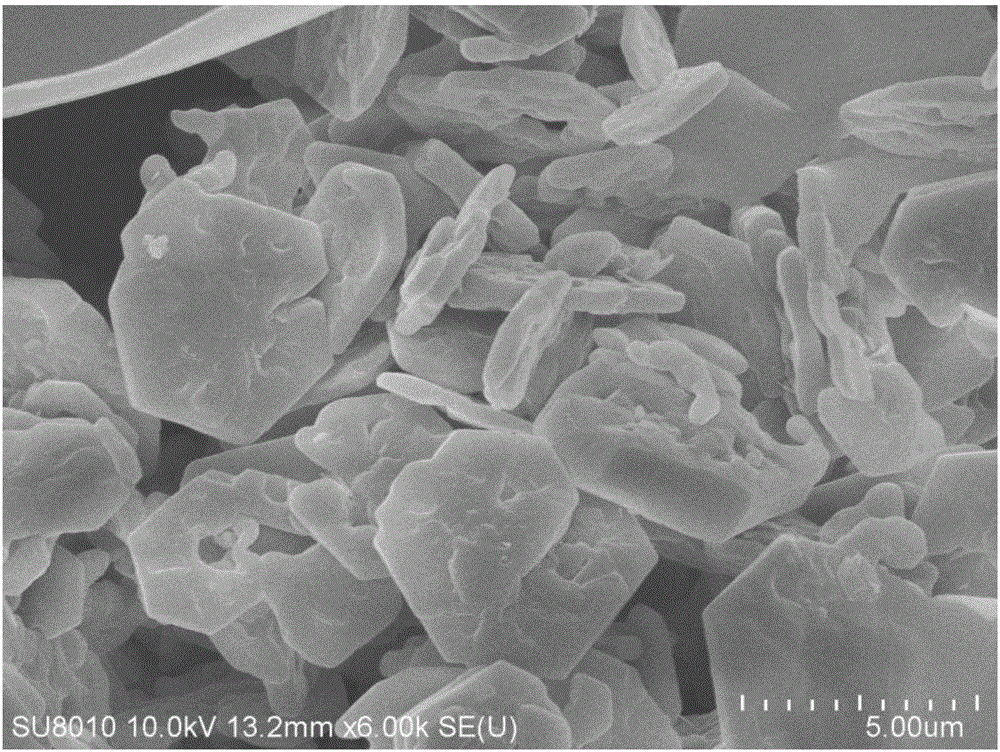
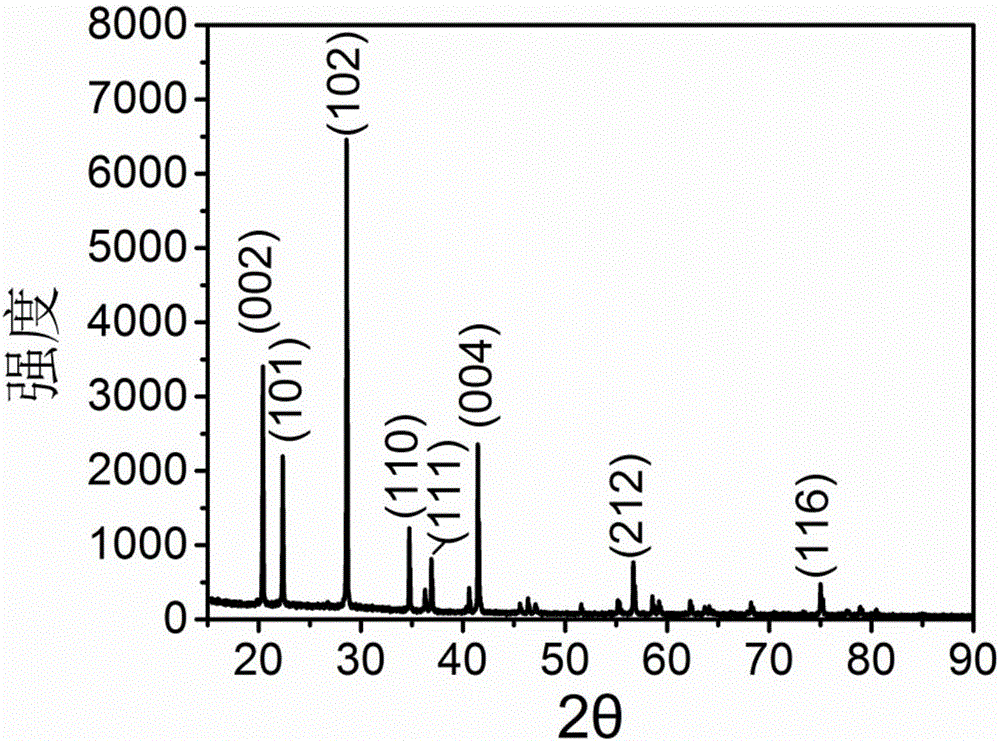
Porous kalsilite and preparation method thereof
A technology for potassium nepheline and palygorskite, which is applied in the field of porous hexagonal potassium nepheline and its preparation, and achieves the effect of enhancing application efficiency and facilitating diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] One aspect of the present invention provides a kind of preparation method of hexagonal kalephine, comprising the steps of:
[0015] S1. Take palygorskite and put it into hydrochloric acid for activation for 2-12 hours, then wash with pure water, dry and grind to obtain substance A for later use;
[0016] Preferably, in step S1, 1-2 g of palygorskite is added per 10 ml of water;
[0017] Preferably, in step S1, the particle size of palygorskite selected is not less than 200 mesh;
[0018] Preferably, in step S1, the hydrochloric acid concentration is 6-8 mol / L;
[0019] S2. Add 2 to 3 g of aluminum nitrate per 20 ml of water, stir evenly, and then add potassium hydroxide or a mixture of potassium hydroxide and sodium hydroxide to make OH in the solution - The concentration is 10-12mol / L, and stirred evenly at room temperature to obtain solution B, then take 1-1.5g of substance A into solution B, stir evenly, and obtain substance C for later use;
[0020] Preferably, i...
Embodiment 1
[0027] S1. Take 10 g of palygorskite with a particle size of 200 mesh and put it into 100 mL of hydrochloric acid with a concentration of 6 mol / L to activate for 12 hours, then wash it with deionized water until there is no chloride ion, dry it at 60 ° C, grind it, and obtain the substance A for use;
[0028] S2. Dissolve 2g of aluminum nitrate in 20ml of water, stir evenly, add 5.6g of potassium hydroxide, and stir evenly at room temperature to obtain solution B, then take 1g of substance A and put it into solution B, stir evenly to obtain substance C spare;
[0029] S3. Put the obtained substance C into a hydrothermal reaction kettle, react at 160°C for 72h, cool to room temperature, centrifuge at a speed of 4000 rpm, wash with pure water, and dry at 60°C to obtain porous hexagonal nephrite .
Embodiment 2
[0031] S1. Take 30 g of palygorskite with a particle size of 300 mesh and put it into 150 mL of hydrochloric acid with a concentration of 8 mol / L for activation for 10 hours, then wash it with deionized water until there is no chloride ion, dry it at 60 ° C, grind it, and obtain substance A for use;
[0032] S2. Dissolve 3g of aluminum nitrate in 20mL of water, stir evenly, add 2.8g of potassium hydroxide and 2g of sodium hydroxide, and stir evenly at room temperature to obtain solution B, then take 1.1g of substance A and put it into solution B, Stir evenly to obtain substance C for later use;
[0033] S3. Put the obtained substance C into a hydrothermal reaction kettle, react at 160°C for 12h, cool to room temperature, centrifuge at a speed of 4000 rpm, wash with pure water, and dry at 60°C to obtain porous hexagonal nephrite .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com