Continuous production method of ethyl chloride
A production method and technology of ethyl chloride, applied in chemical instruments and methods, organic chemistry, disproportionation separation/purification of halogenated hydrocarbons, etc., can solve problems such as many by-products, complicated separation and purification system, and increased water volume, and achieve reaction The effect of stabilizing the system, accelerating the reaction rate, and accelerating the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
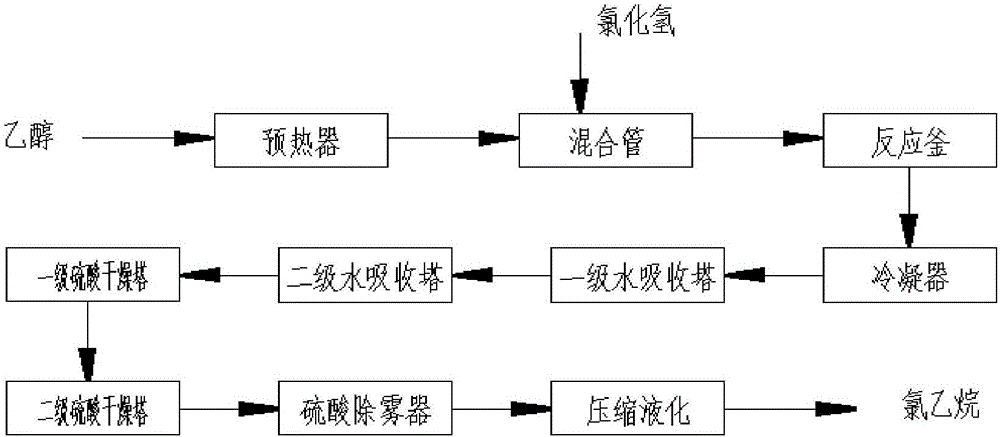
[0028] Add 6000L zinc chloride aqueous solution with a mass percentage of 50% in a 10000L enamel reaction kettle, and the ethanol flows through the preheater at a flow rate of 300Kg / h. Mixing tube, hydrogen chloride gas is passed into the mixing tube at a flow rate of 250Kg / h. After the ethanol and hydrogen chloride are fully mixed, they are dispersed into the reaction system through the distributor, and the dehydration reaction starts under the action of zinc chloride. The temperature and pressure of the reaction system are maintained at 130 ~135°C and 0.10~0.13MPa, the gas phase system is condensed by the condenser to form gas phase crude ethyl chloride, and the condensate is returned to the reactor. The crude ethyl chloride is refined through two-stage water absorption tower, two-stage sulfuric acid drying tower and sulfuric acid demister, compressed and liquefied, and the finished product of ethyl chloride with a flow rate of 400Kg / h is obtained, with a purity of 99.93%, a ...
Embodiment 2
[0031] Add 6000L zinc chloride aqueous solution with a mass percentage of 60% in a 10000L enamel reaction kettle, and the ethanol flows through the preheater at a flow rate of 300Kg / h. Mixing tube, hydrogen chloride gas is passed into the mixing tube at a flow rate of 250Kg / h. After the ethanol and hydrogen chloride are fully mixed, they are dispersed by the distributor and enter the reaction system. The dehydration reaction starts under the action of zinc chloride. The temperature and pressure of the reaction system are maintained at 130~135℃ and 0.10~0.13MPa, the gas phase system is condensed by the condenser to form gas phase crude ethyl chloride, and the condensate is returned to the reactor. The crude ethyl chloride is refined through two-stage water absorption tower, two-stage sulfuric acid drying tower and sulfuric acid demister, compressed and liquefied, and the finished product of ethyl chloride with a flow rate of 400Kg / h is obtained, with a purity of 99.95%, a water ...
Embodiment 3
[0034] Add 6000L zinc chloride aqueous solution with a mass percentage of 70% in a 10000L enamel reaction kettle, and the ethanol flows through the preheater at a flow rate of 300Kg / h. Mixing tube, hydrogen chloride gas is passed into the mixing tube at a flow rate of 250Kg / h. After the ethanol and hydrogen chloride are fully mixed, they are dispersed by the distributor and enter the reaction system. The dehydration reaction starts under the action of zinc chloride. The temperature and pressure of the reaction system are maintained at 140~145℃ and 0.10~0.13MPa, the gas phase system is condensed by the condenser to form gas phase crude ethyl chloride, and the condensate is returned to the reactor. The crude ethyl chloride is refined through a two-stage water absorption tower, a two-stage sulfuric acid drying tower and a sulfuric acid demister, compressed and liquefied to obtain a finished product of ethyl chloride with a flow rate of 400Kg / h, a purity of 99.98%, a water content ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com