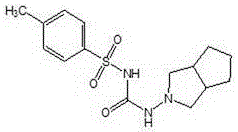
Preparation method of gliclazide in green synthesis technology
A green synthesis and process technology, applied in the direction of organic chemistry, can solve the problems of difficult separation and purification of double salts, high environmental treatment costs, etc., and achieve the effects of improving reaction yield, facilitating industrial production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
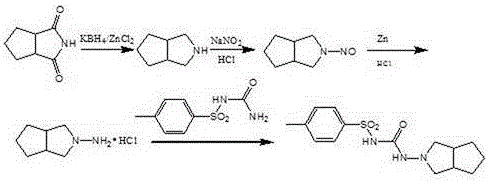
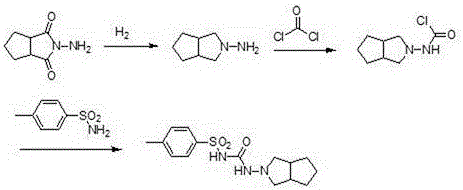
[0030] (1) Preparation of N-amino-3-azabicyclo[3,3,0]octane
[0031] Add 154g (1.0mol) of N-amino-1,2-cyclopentanedicarboximide, 700g of acetic acid, and copper-chromium catalyst (Cr-Cu atomic ratio 0.14) into a 2L high-pressure reactor with a stirring and heating device 4.6g, after stirring evenly, replace the air in the reactor with nitrogen, then replace the nitrogen in the reactor with hydrogen, and finally pressurize it to 1.5MPa with hydrogen, heat the reactor to 60°C, and react for 4 hours. After the reaction, Cool to room temperature, filter to remove the catalyst, concentrate the filtrate under negative pressure to remove the solvent acetic acid, add the residue to tetrahydrofuran, and use triethylamine to adjust the pH value of the solution to 6.5, filter, and distill the solvent from the filtrate to obtain a light yellow liquid N-amino-3-nitrogen Heterobicyclo[3,3,0]octane 114.28 (0.906mol) g, the yield was 90.6%, the crude product was directly used in the next reac...
Embodiment 2
[0037] (1) Preparation of N-amino-3-azabicyclo[3,3,0]octane
[0038]Add 154g (1.0mol) of N-amino-1,2-cyclopentanedicarboximide, 700g of acetic acid, and copper-chromium catalyst (Cr-Cu atomic ratio 0.2) into a 2L high-pressure reactor with a stirring and heating device 4.6g, after stirring evenly, replace the air in the reactor with nitrogen, then replace the nitrogen in the reactor with hydrogen, and finally pressurize it to 2MPa with hydrogen, raise the temperature of the reactor to 80°C, and react for 4 hours. After the reaction, cool To room temperature, filter to remove the catalyst, concentrate the filtrate under negative pressure to remove the solvent acetic acid, add tetrahydrofuran to the residue, and adjust the pH of the solution to 6.8 with triethylamine, filter, and distill the solvent from the filtrate to obtain a pale yellow liquid N-amino-3-azabicyclo [3,3,0]octane 115.3g (0.92mol), the yield was 91.37%, the crude product was directly used in the next reaction w...
Embodiment 3
[0044] (1) Preparation of N-amino-3-azabicyclo[3,3,0]octane
[0045] Add 154g of N-amino-1,2-cyclopentanedicarboximide, 700g of acetic acid, and 4.6g of copper-chromium catalyst (Cr-Cu atomic ratio 0.26) into a 2L high-pressure reactor with a stirring and heating device, and stir After uniformity, replace the air in the reactor with nitrogen, then replace the nitrogen in the reactor with hydrogen, and finally pressurize it to 2.5 MPa with hydrogen, heat the reactor to 90°C, and react for 4 hours. After the reaction, cool to room temperature. The catalyst was removed by filtration, the filtrate was concentrated under negative pressure to remove the solvent acetic acid, the residue was added to tetrahydrofuran, and the pH of the solution was adjusted to 7 with triethylamine, filtered, and the solvent was distilled from the filtrate to obtain a pale yellow liquid N-amino-3-azabicyclo[3, 3,0] Octane 118.6g, the yield was 93.97%, the crude product was directly used in the next reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com