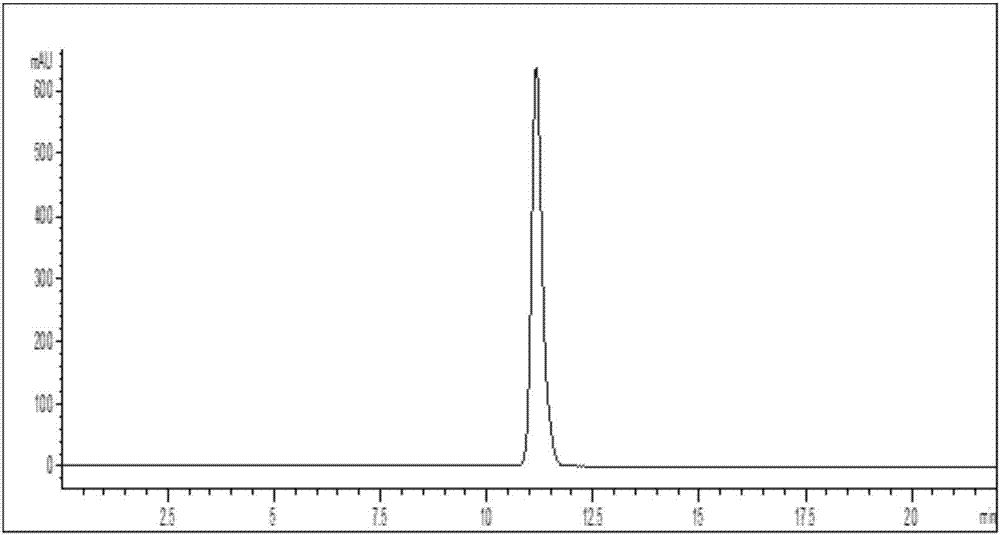
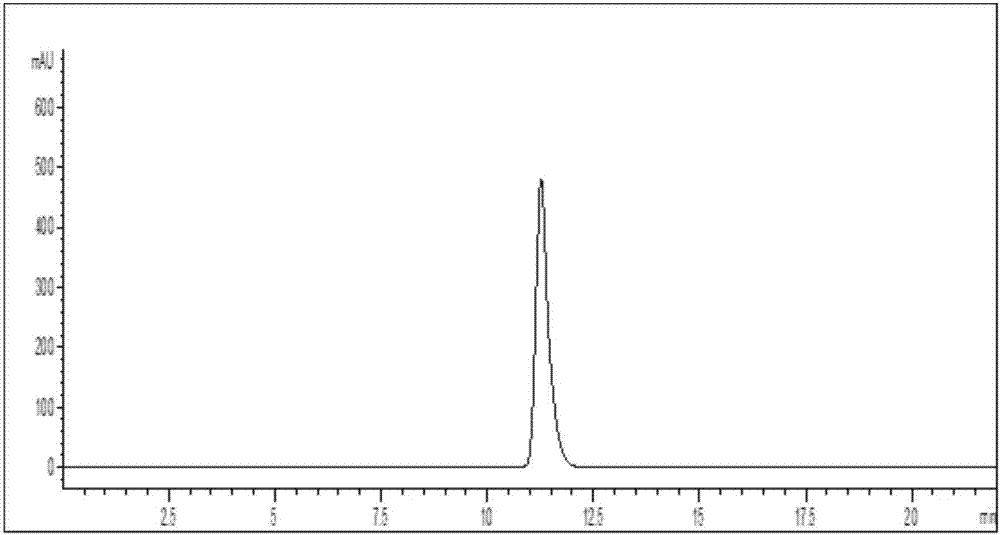
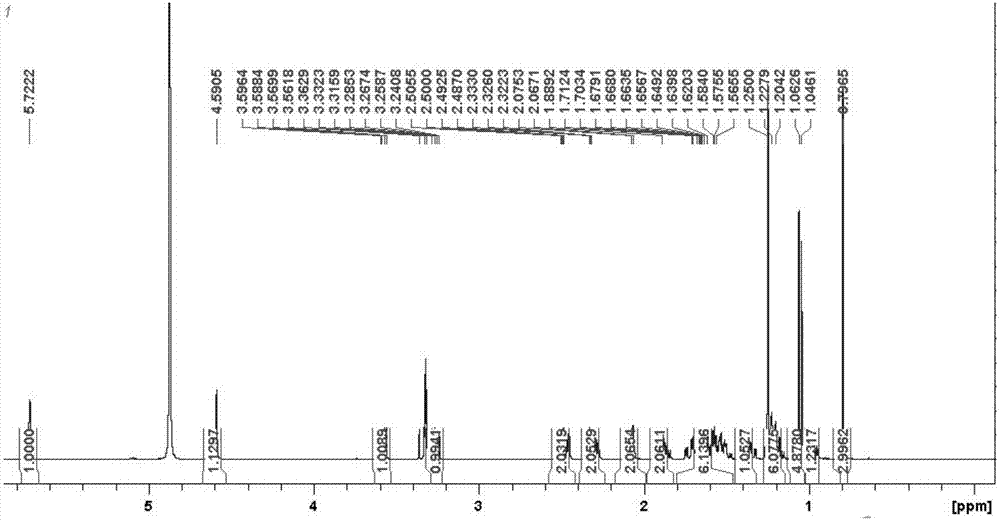
Mycobacterium sp. strain for biosynthesis of 20-hydroxy-23,24-bisnorchol-4-ene-3-one and synthetic method thereof
A technology of biosynthesis and mycobacteria, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of long transformation time, many by-products, low transformation efficiency, etc., and achieve easy separation and purification, reduce Pollution, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Obtaining strain Mycobacterium sp.FJH0105-16 by mutagenesis
[0022] 1.1 Protoplast preparation
[0023] The original strain is Mycobacterium sp. 1229-3, which transforms phytosterols into the main product androst-4-ene-3,17-dione, and the by-product 20-hydroxy-23,24-dicholesterol -4-en-3-one, the ratio of the two is 2:1. The strain was screened from the soil of Tianjin Yihai Kerry Oil Company. Melt the glycerol tube of the preserved original bacterial strain and inoculate it in the seed medium with an inoculum of 2%, cultivate it at 30°C and 200rpm for 36h, transfer the seed solution to the seed medium containing 12.5g / l glycine with a 5% inoculum , cultivated for 24h, 6000rpm, and collected the bacteria for 10min. Wash the cells twice with hypertonic buffer (plus 0.2% Tween 80). Resuspend the bacteria in hyperosmotic enzymatic hydrolysis solution, the concentration of lysozyme is 50mg / ml, enzymatic hydrolysis for 3h, 8000rpm, 20min centrifugation to rem...
Embodiment 2
[0038] Embodiment 2: The method that the mycobacterium provided by the invention transforms phytosterols into 20-hydroxyl-23,24-dinorchol-4-en-3-one
[0039] 2.1 Inclined seed culture
[0040] Mycobacterium sp. FJH0105-16 (Mycobacterium sp. FJH0105-16), the deposit number is CGMCCNO.13234, was cultured at 30° C. for 3 days. The formula of the slant medium is 200g / l potato, 20g / l glucose, 15g / l agar, and natural pH.
[0041] 2.2 Primary seed cultivation
[0042] The bacterial colonies cultivated by the above slant seeds were inserted into the sterilized liquid seed medium, and cultured at 30° C. and 200 rpm for 2 days to obtain seed liquid. The formula of described liquid seed culture medium is, glucose 6g / l, yeast extract 10g / l, Na 2 HPO 4 4.5g / l, KH 2 PO 4 3.4 g / l, 0.2% Tween 80, pH 7.0.
[0043] 2.3 Fermentation and transformation in fermenter to produce 20-hydroxy-23,24-dinorchol-4-en-3-one
[0044] Inoculate the seed liquid obtained in step 2.2 into the sterilized ...
Embodiment 3
[0047] Embodiment 3: The method that the mycobacterium provided by the present invention transforms phytosterols into 20-hydroxyl-23,24-dinorchol-4-en-3-one
[0048] 3.1 Inclined seed culture
[0049] Mycobacterium sp. FJH0105-16 (Mycobacterium sp. FJH0105-16), the deposit number is CGMCCNO.13234, was cultured at 30° C. for 3 days. The medium components are as shown in Example 2.
[0050] 3.2 Primary seed cultivation
[0051] The bacterial colonies cultivated by the above slant seeds were inserted into the sterilized liquid seed medium, and cultured at 30° C. and 200 rpm for 2 days to obtain seed liquid. The components of the liquid seed medium are as shown in Example 2.
[0052] 3.3 Fermentation and transformation in fermenter to produce 20-hydroxy-23,24-dinorchol-4-en-3-one
[0053] Inoculate the seed liquid obtained in step 3.2 into the sterilized transformation medium, the inoculum size is 10%, the transformation temperature is 30°C, the rotation speed is 400rpm, the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com