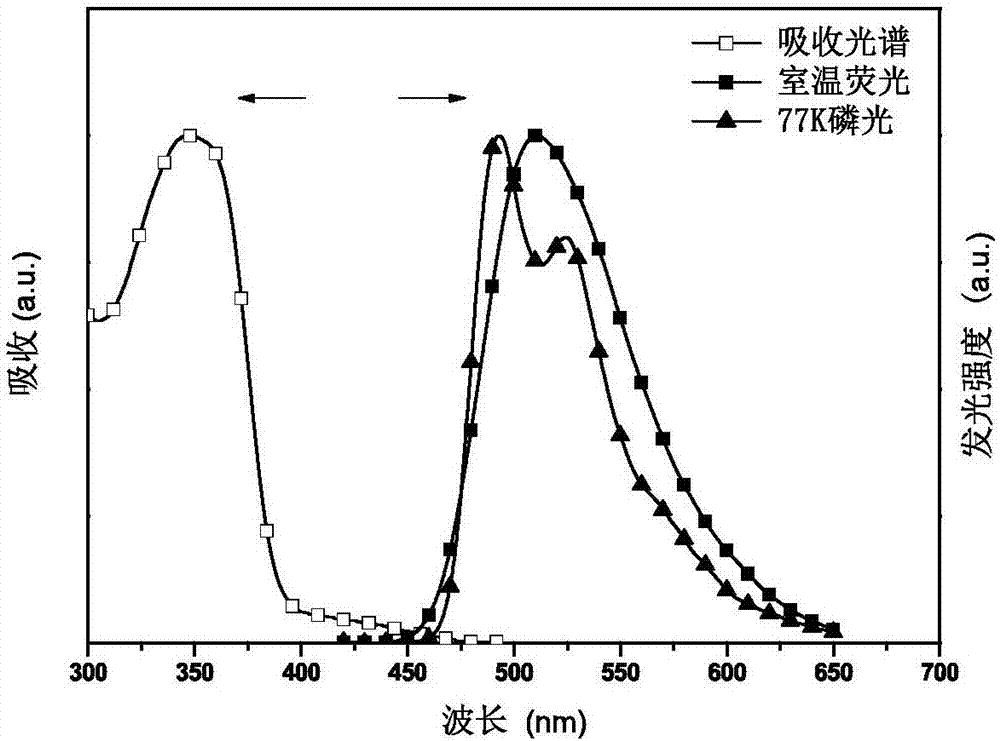
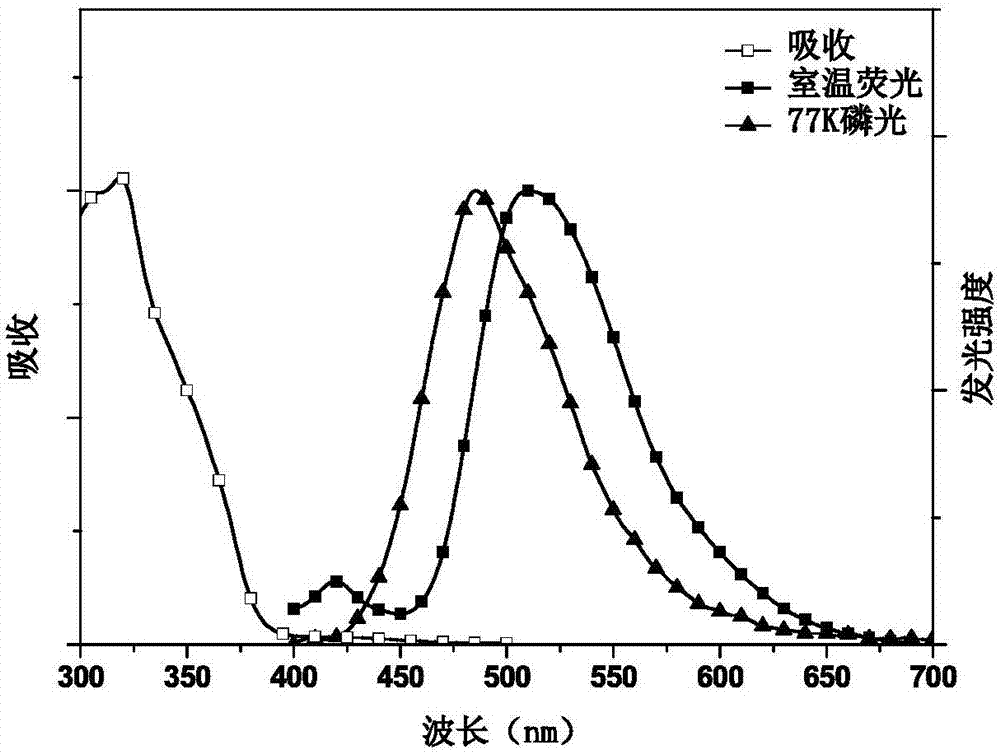
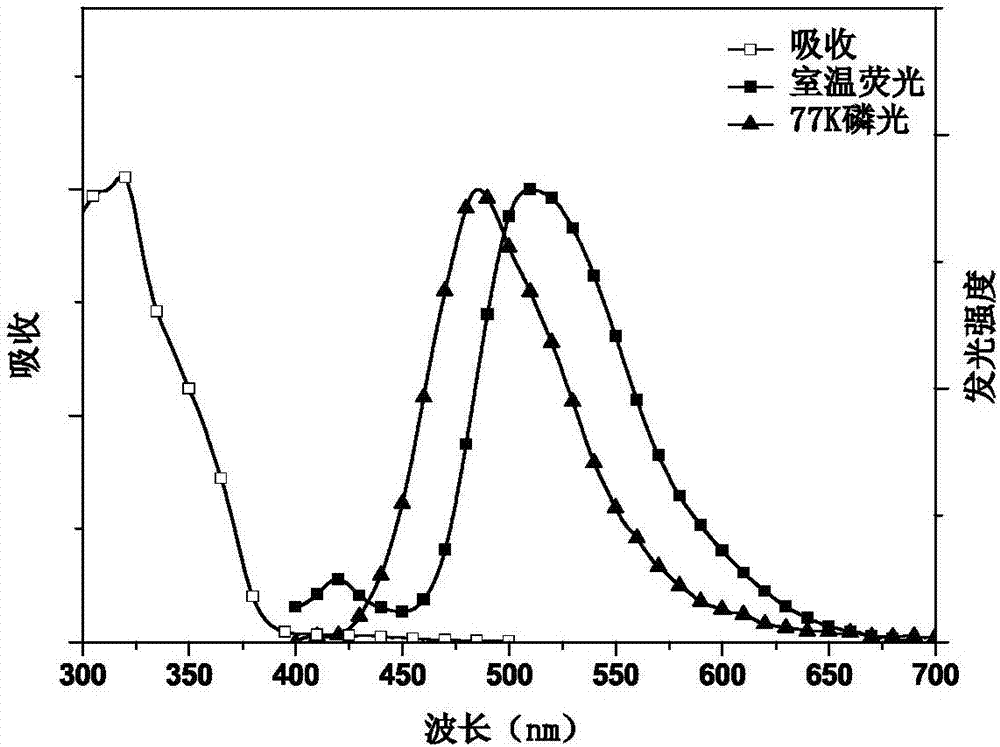
Conjugated polymer with side chain containing triazine group as well as preparation method and application of conjugated polymer
A technology of conjugated polymer and triazine group, applied in the field of conjugated polymer and its preparation, can solve problems such as efficiency roll-off, and achieve the effects of improving performance, simple preparation method and improving current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The present invention also provides a preparation method of the conjugated polymer containing triazine groups in the side chain of the present invention, comprising:
[0062] Copolymerize the compound with the structure of formula (II), the compound with the structure of formula (III) and the compound with the structure of formula (IV) to obtain the compound with the structure shown in formula (I);
[0063]
[0064] Among them, R 1 , R 3 independently selected from C1-C30 alkyl, C1-C30 alkoxy, C6-C35 unsubstituted aryl or C6-C35 substituted aryl;
[0065] R 2 C1-C30 alkyl, C1-C30 alkoxy, C6-C35 unsubstituted aryl, C6-C35 unsubstituted phenol, C6-C35 substituted aryl or C6-C35 substituted phenol;
[0066] x is 0.001
[0067] n is 2-200.
[0068] In the present invention, the present invention will have the compound of formula (II) structure, the compound of formula (III) structure and the compound of formula (IV) structure to copolymerize, obtain the comp...
Embodiment 1
[0073] Example 1: Synthesis of Polymer PCz-HAPT35%
[0074] 1) Preparation of unbrominated formula (II) structural compound
[0075] The preparation process is shown in the following formula:
[0076]
[0077] The specific steps are:
[0078] 9,9-dihexyl-9,10-dihydroacridine (2.0g, 3.7mmol), 4,6-di-tert-butyl-2-p-bromophenyl-1,3,5-triazine (1.3 g, 3.7mmol), Pd2 (dba) 3 (0.003g, 0.04mmol), DPPF (0.005g, 0.08mmol), sodium tert-butoxide (0.71g, 7.4mmol) were added in a 100ml three-necked flask, and 20ml of dry Toluene was pumped out, protected by argon, condensed at 80°C for 20 hours, cooled to room temperature, extracted with water and dichloromethane, the organic phase was rotary evaporated, and separated by a column to obtain 2.0 g of the product with a yield of 90%.
[0079]The obtained product is carried out nuclear magnetic resonance detection, and its hydrogen spectrum is: 1 H NMR (400MHz, CDCl 3 )68.84(d, J=8.3Hz, 2H), 7.40(d, J=8.3Hz, 2H), 7.30(m, 2H), 6.94-6.81(...
Embodiment 2
[0091] Example 2: Synthesis of Polymer PCz-HAPT25%
[0092]
[0093] The specific steps are:
[0094] 2,7-Dibromo-9,9-dihexyl-10-(6-(4',6'-di-tert-butyl-1,3,5-triazine))phenyl-9,10-dihydro Acridine (0.194g, 0.25mmol), 3,6-dibromo-9-heptadecylcarbazole (0.141g, 0.25mmol), 3,6-dipinacol borate-9-heptadecane Carbazole (0.329g, 0.5mmol), bis(tri-o-methylphenylphosphine) palladium dichloride (0.004g, 0.005mmol), and potassium phosphate solution (2M, 1.6ml) were added to a 50ml one-necked flask In the middle, pump and exchange gas, protect with argon, add deoxygenated tetrahydrofuran (8ml), reflux reaction at 80°C for 24h; inject phenylboronic acid (0.015g, 0.1mmol) dissolved in 2ml tetrahydrofuran into the reaction solution, react for 6h, and then Bromobenzene (0.1ml) dissolved in 2ml of tetrahydrofuran was injected into the reaction solution and reacted for 6h; Diethylcarbamothiocarbamate (1g) dissolved in 20ml of water was added to the reaction solution and continued to stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com