Application of apocynin nitrone in preparing medicine for preventing and treating asthma
A technology of berberine and nitrone, which is applied in the field of ferberine nitrone in the field of preparing asthma prevention and treatment drugs, can solve the problem that the drug efficacy needs to be improved, and achieve the effects of enhancing the antioxidant effect and making up for the deficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
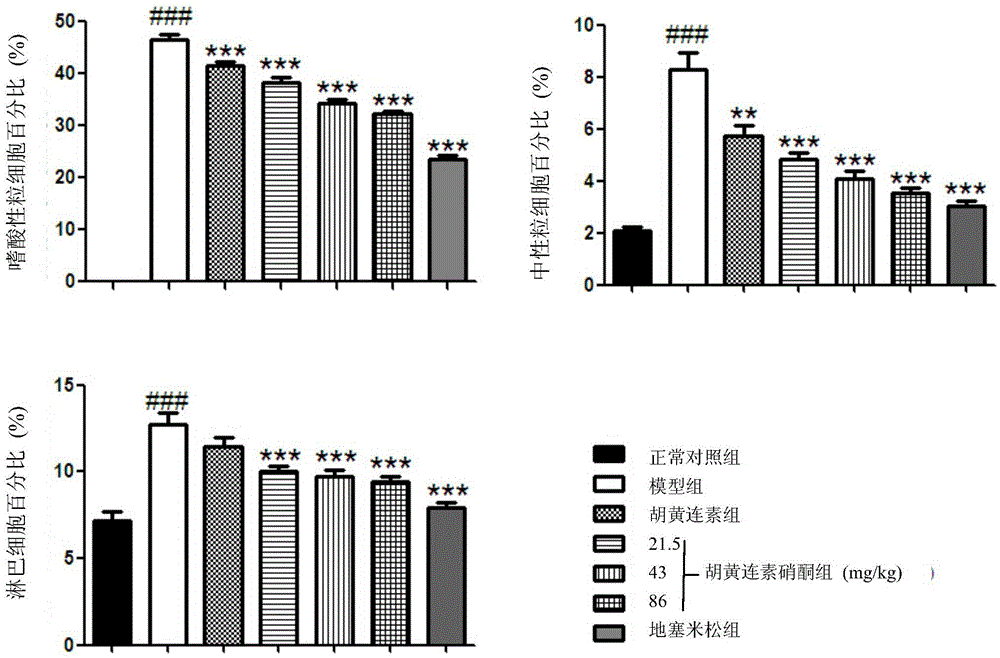
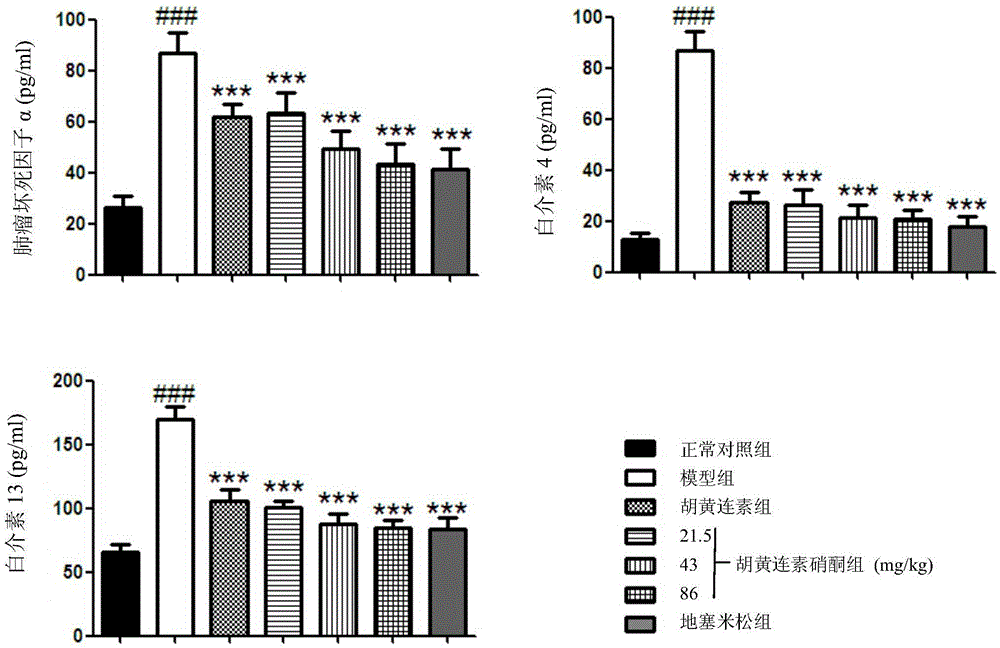
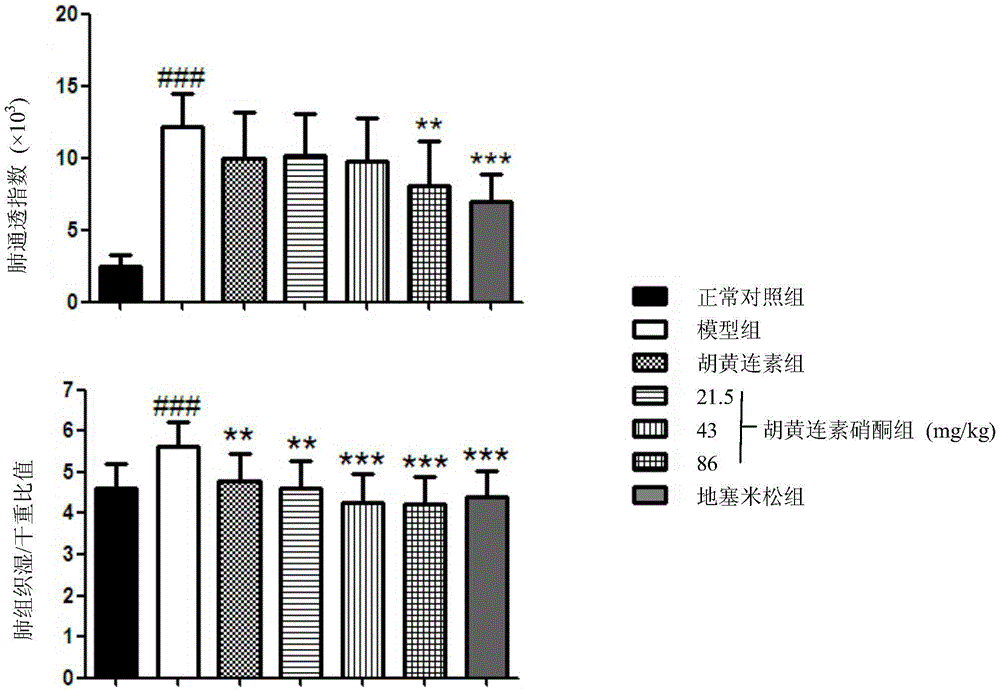
[0032] Female BALB / c mice, weighing 20-25g, aged 6-8 weeks, were randomly divided into normal control group, OVA model group, berberine (Apo) group, berberine nitrone (AN-1) low and medium , high-dose group, positive drug dexamethasone (Dex) group, 11 in every group. Placed in an environment at 23±2°C with a lighting time of 12h / day, and the experiment began after the animals had adapted to the environment for one week. From the first day of the experiment, except for the normal control group, animals in each group were injected intraperitoneally with 200 μl of phosphate buffered saline (PBS) containing 10 μg of ovalbumin (OVA) and 1 mg of aluminum hydroxide, once every 7 days for 3 weeks. The normal control group was replaced by an equal volume of PBS. From the 21st day, except for the normal control group, the mice in each group were placed in a transparent airtight container, and the stimulation solution (PBS containing 20g / L OVA) was atomized for stimulation, once a day, ...
Embodiment 2
[0040] Female BALB / c mice, weighing 20-25g, aged 6-8 weeks, were randomly divided into normal control group, OVA model group, berberine (Apo) group, berberine nitrone (AN-1) low and medium , high-dose group, positive drug dexamethasone (Dex) group, 11 in every group. Placed in an environment at 23±2°C with a lighting time of 12h / day, and the experiment began after the animals had adapted to the environment for one week. From the first day of the experiment, except for the normal control group, animals in each group were injected intraperitoneally with 200 μl of phosphate buffered saline (PBS) containing 10 μg of ovalbumin (OVA) and 1 mg of aluminum hydroxide, once every 7 days for 3 weeks. The normal control group was replaced by an equal volume of PBS. From the 21st day, except for the normal control group, the mice in each group were placed in a transparent airtight container, and the stimulation solution (PBS containing 20g / L OVA) was atomized for stimulation, once a day, ...
Embodiment 3
[0043]Female BALB / c mice, weighing 20-25g, aged 6-8 weeks, were randomly divided into normal control group, OVA model group, berberine (Apo) group, berberine nitrone (AN-1) low and medium , high-dose group, positive drug isoproterenol group, 11 rats in each group. Placed in an environment at 23±2°C with a lighting time of 12h / day, and the experiment began after the animals had adapted to the environment for one week. From the first day of the experiment, except for the normal control group, animals in each group were intraperitoneally injected with 200 μl of phosphate buffered saline (PBS) containing 10 μg of ovalbumin (OVA) and 1 mg of aluminum hydroxide, once every 7 days for 3 weeks. The normal control group was replaced by an equal volume of PBS. From the 21st day, except for the normal control group, the mice in each group were placed in a transparent airtight container, and the stimulation solution (PBS containing 20g / LOVA) was atomized to challenge, once a day, 30min e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com