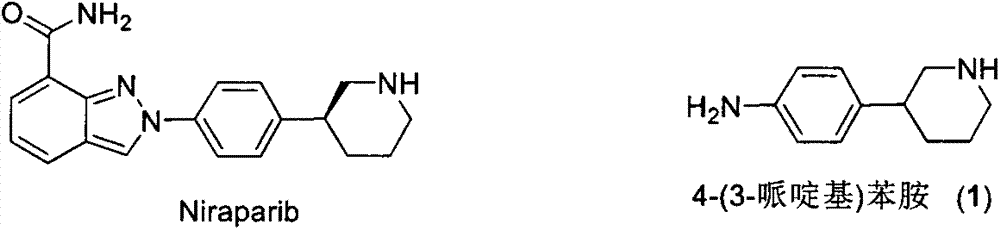
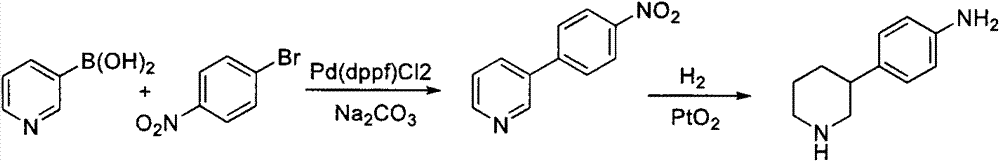
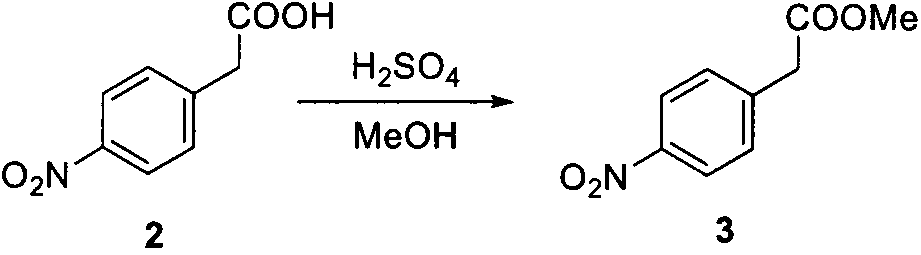
Preparation method of 4-(3-piperidino) aniline and tartrate thereof
A technology of tartrate and methyl nitrophenylacetate, applied in directions such as organic chemistry, can solve the problems of expensive reagents and high equipment requirements, and achieve the effects of low price, low cost and reduction of reaction costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be further described below through the examples, but the examples do not limit the protection scope of the present invention.
[0031] 1. Preparation of methyl p-nitrophenylacetate (3)
[0032] Dissolve 20g (0.11mol) of p-nitrobenzoic acid in 300ml of methanol, add 2ml of concentrated sulfuric acid, heat up to reflux after addition, and react for 2 hours. Distill off part of the solvent under reduced pressure, add saturated aqueous sodium bicarbonate solution to the residue to adjust the pH to 7-8, add 200ml of water, extract with ethyl acetate (100ml×3), combine the organic phases, and wash with saturated brine (100ml×2) , dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 21.2 g of light yellow needle-like crystals. The crude product was used directly in the next step without purification.
[0033] 2. Preparation of methyl 4-cyano-2-(4-nitrophenyl)butyrate (4)
[0034] The crude product from the previou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com