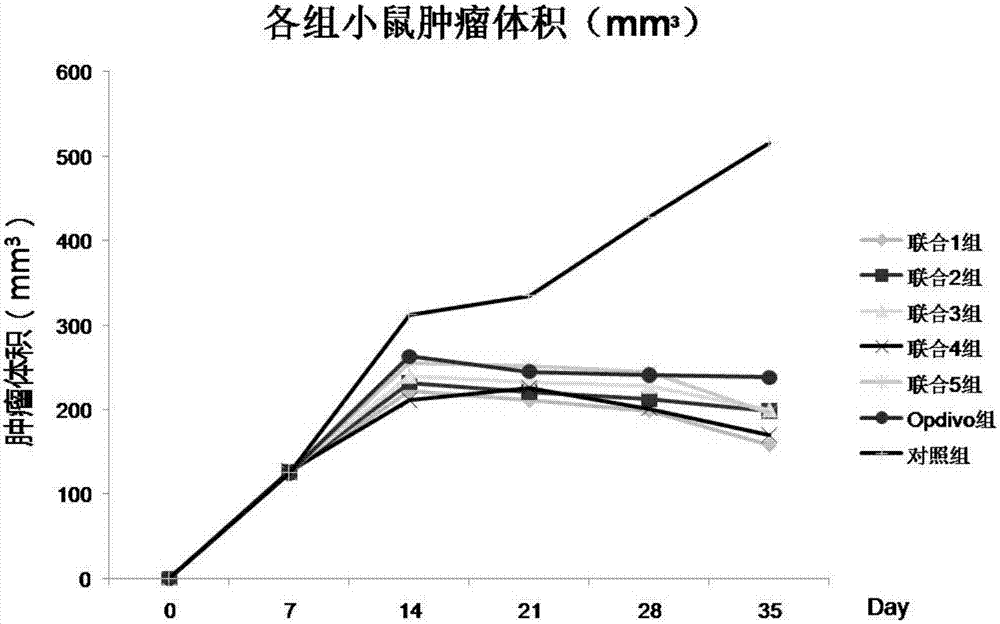
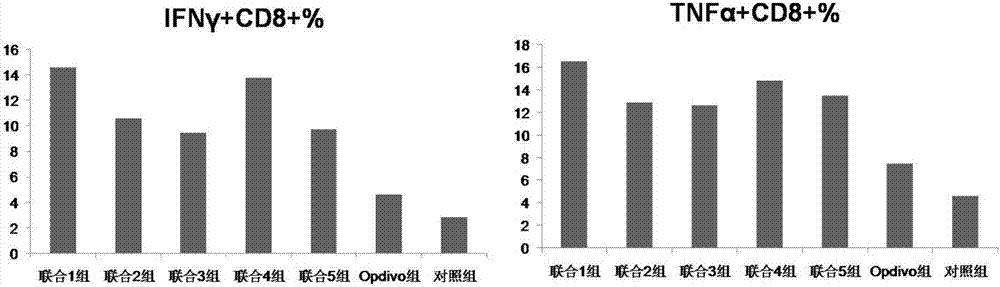
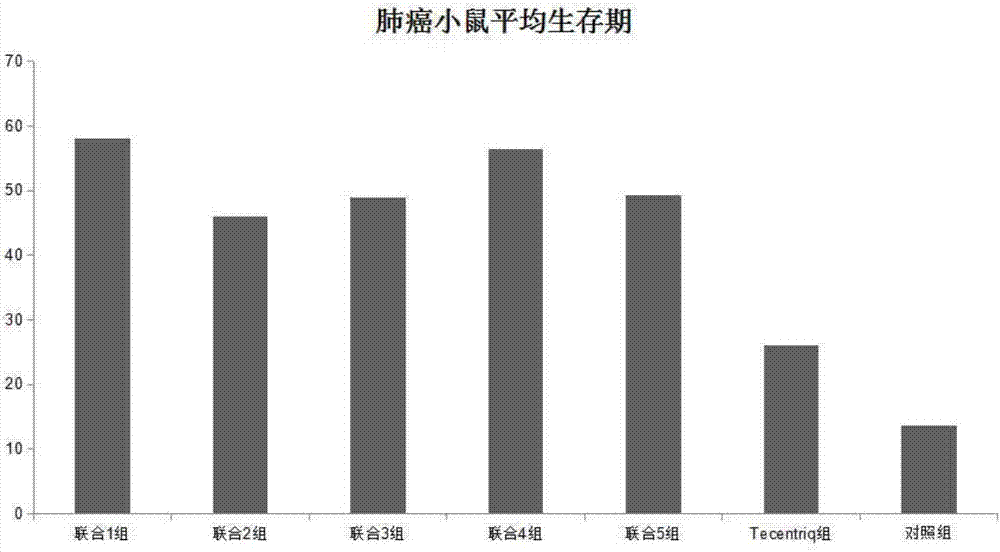
Application of chlorogenic acid and derivative in preparing sensitizer for tumor immunotherapeutic drug
A technology of immunotherapeutic drugs and sensitizers, which is applied in the field of medicine, can solve problems such as drug resistance and non-response, and achieve the effect of promoting proliferation and broad clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Preparation of chlorogenic acid and derivatives thereof
[0048]For the preparation method of chlorogenic acid, see Example 1 of patent ZL2005100207065, which was independently synthesized by Sichuan Jiuzhang Biotechnology Co., Ltd.; both neochlorogenic acid and cryptochlorogenic acid were purchased from Chengdu Best Reagent Co., Ltd.; 3-O-feruloylquinic acid and 3-O-isoferuloylquinic acid: using the chlorogenic acid raw material provided by Sichuan Jiuzhang Biotechnology Co., Ltd. to synthesize 3-O-feruloylquinic acid and its isomer 3-O-isoferuloylquinic acid Nicotinic acid, the synthesized sample was roughly separated by an open ODS column, and then purified by semi-preparative liquid phase, and the detected purity was 96.1% and 99.3%, respectively.
[0049] 1. Instruments and materials
[0050] 1.1 Instruments and equipment
[0051] Linear ion trap-Fourier ion cyclotron resonance mass spectrometer (Thermo Scientific LTQ FT), the product of American The...
Embodiment 2
[0109] The preparation of embodiment 2 chlorogenic acid and derivative capsules thereof
[0110] 1. Prescription
[0111] Table 3 The prescription of chlorogenic acid and derivative capsules thereof of the present invention
[0112]
[0113] 2. Preparation method
[0114] According to the above-mentioned prescription quantity, mix the main ingredient and chitosan respectively and dissolve in the acetic acid solution whose volume concentration is 3% to obtain the chitosan solution of each compound, stand-by; add Span 80 into soybean oil according to the prescription quantity , after stirring evenly, the mixed solution is obtained, which is ready for use; 5 parts of chitosan solution prepared are slowly added dropwise to the mixed solution, and continuously stirred to obtain a W / O emulsion; Glutaraldehyde with a concentration of 25% was left to stand and solidified and cross-linked for 2 hours, then the oil phase was removed and filtered through a Buchner funnel with a 0.45...
Embodiment 3
[0115] Embodiment 3 Preparation of Chlorogenic Acid and Its Derivatives Freeze-dried Powder Injection
[0116] 1. Prescription
[0117] Table 4 The prescription of chlorogenic acid and its derivatives freeze-dried powder injection
[0118]
[0119] 2. Preparation method
[0120] Under strict aseptic conditions, take by weighing 10 g of chitosan of the above-mentioned prescriptions, dissolve it with 3% acetic acid solution to form a chitosan solution, and weigh the main ingredients according to the prescription and dissolve them in the above-mentioned chitosan solution respectively. In sugar solution, form the chitosan solution of each principal agent, the solution after dissolving is left standstill for a period of time and carry out spray drying after air bubble is removed, promptly obtains the chitosan microsphere with good fluidity, each obtained The chitosan microspheres of chlorogenic acid and the like are filled into powder injections to obtain corresponding freeze-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com