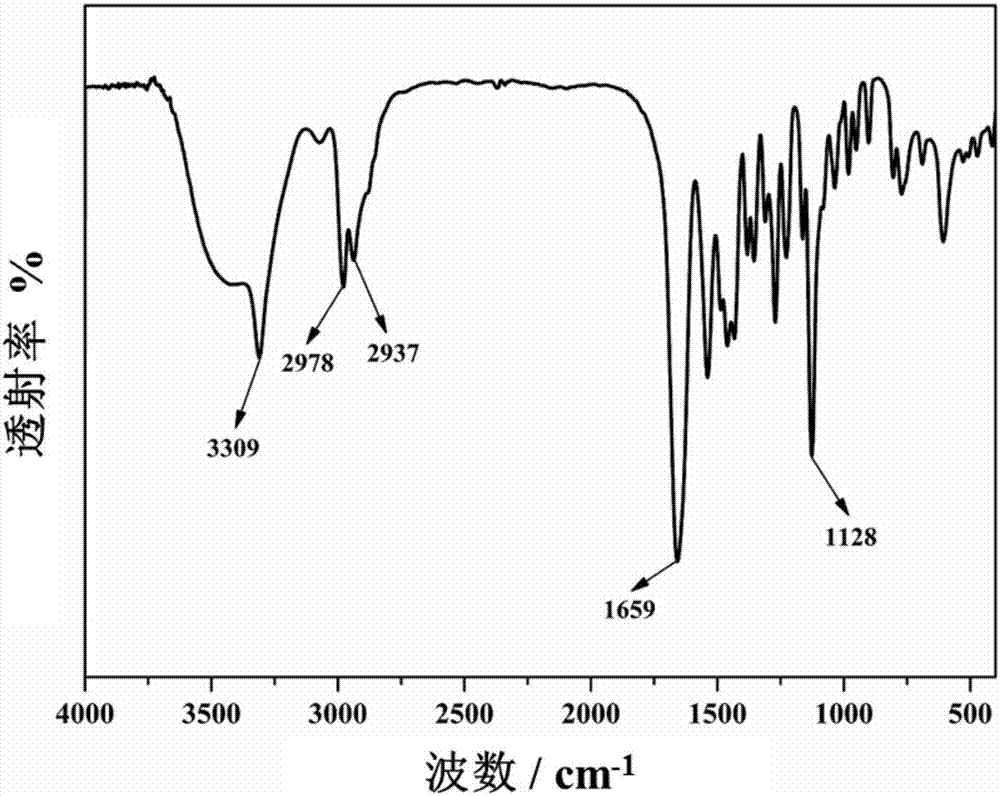
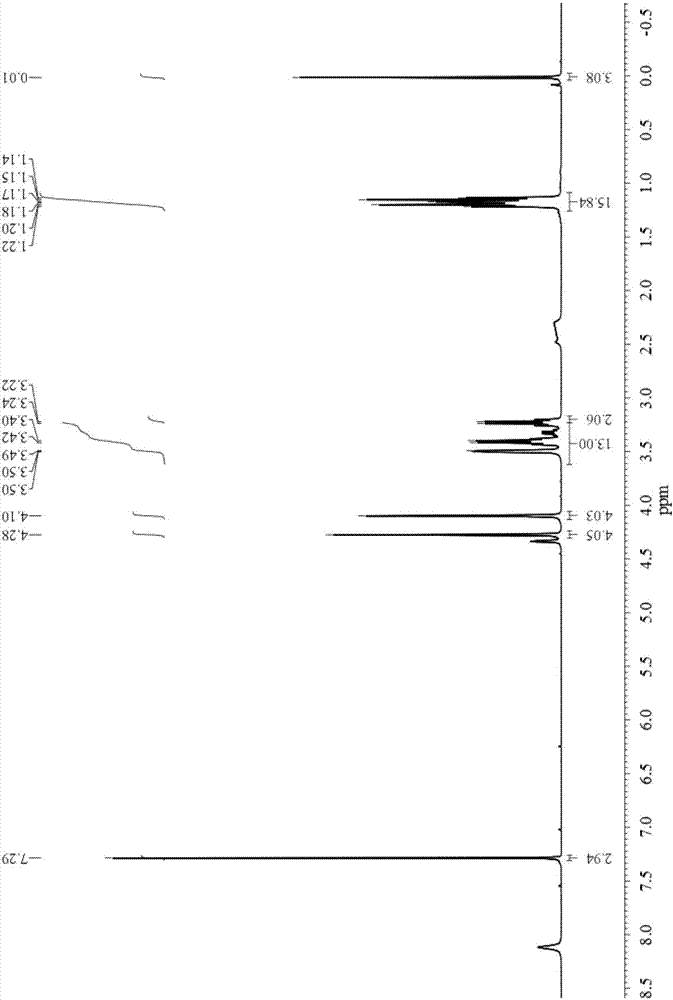
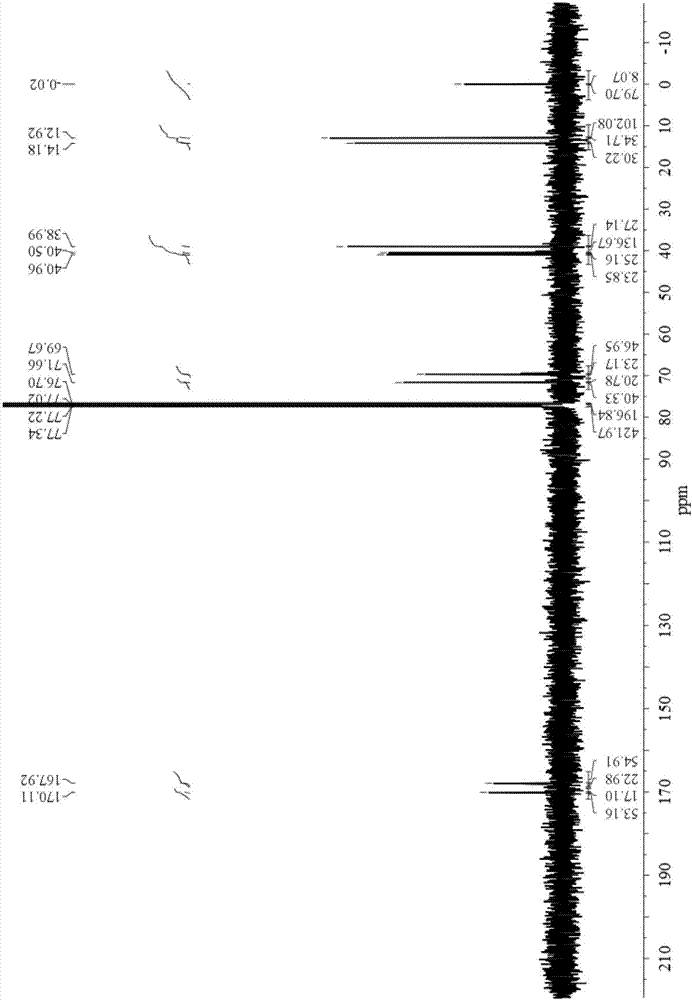
Bisdiglycolamide ligand and preparation method thereof and lanthanum serial/actinium serial separation and extraction system containing bisdiglycolamide ligand
A bisglyceride and ligand technology, which is applied in the field of nuclear fuel cycle and nuclear waste treatment, can solve the problems of unsatisfactory and insufficient separation factor, achieve strong practicability, improve radiation resistance stability, and expand the scope of application Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This example prepares N,N,N"',N"'-tetraethyl-N',N"-ethylenediyl-bisdiglyceramide (TEE-BisDGA) with the structural formula (10). The route looks like this:
[0050]
[0051] The preparation process of the above-mentioned TEE-BisDGA comprises the following steps:
[0052] (1) Diglycolic anhydride (2.9g, 0.025mol) was added to 40mL of tetrahydrofuran, and placed in an ice-water bath at 0°C, and diethylamine (1.8g, 0.025mol) was added dropwise while stirring. After the addition was complete, remove In an ice-water bath, react at 25°C for 3 hours under stirring conditions; then rotary steam the product obtained from the reaction to remove tetrahydrofuran, and then add dropwise an aqueous solution of hydrochloric acid with a volume concentration of 50% to the product obtained from the reaction of removing tetrahydrofuran, and white crystals are precipitated. Suction filtration, and recrystallization and purification using a mixed solvent of methanol / water with a volume ra...
Embodiment 2
[0063] This example prepares N,N,N"',N"'-tetraethyl-N',N"-propanediyl-bisdiglyceramide (TEP-BisDGA) with structural formula (11). The route looks like this:
[0064]
[0065] The preparation process of above-mentioned TEP-BisDGA comprises the following steps:
[0066] (1) Diglycolic anhydride (2.9g, 0.025mol) was added to 40mL 1,4-dioxane, and placed in an ice-water bath at 2°C, diethylamine (2.0g, 0.027mol) was added dropwise while stirring , after the dropwise addition, the ice-water bath was removed, and the reaction was carried out at 25°C for 2.5 hours under stirring conditions; In the obtained product, a hydrochloric acid aqueous solution with a volume concentration of 50% was added dropwise, and white crystals were precipitated. The white crystals were subjected to suction filtration, and recrystallized and purified using a mixed solvent of methanol / water with a volume ratio of 1:1 to obtain N , N-diethyl-3-oxoglutaric acid;
[0067] (2) N, N-diethyl-3-oxoglutaric...
Embodiment 3
[0071] In this example, N,N"'-dimethyl-N,N"'-diethyl-N',N"-butanediyl-bisdiglyceramide (DMDEB-BisDGA) with structural formula (12) was prepared. ), and its synthetic route is as follows:
[0072]
[0073] The preparation process of above-mentioned DMDEB-BisDGA comprises the following steps:
[0074] (1) Diglycolic anhydride (2.9g, 0.025mol) was added to 30mL of dichloromethane, and placed in an ice-water bath at 4°C, and methylethylamine (1.5g, 0.025mol) was added dropwise while stirring. After the dropwise addition, Remove the ice-water bath, and react at 25°C for 2.5 hours under stirring conditions; then rotary steam the product obtained from the reaction to remove dichloromethane, and then add dropwise an aqueous solution of hydrochloric acid with a volume concentration of 50% to the reaction product obtained by removing dichloromethane, and a white Crystals, the white crystals were suction filtered, and recrystallized and purified using a mixed solvent of methanol / wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com