A fluorescent probe with aggregation-induced luminescent properties and its preparation method and use
A technology of aggregation-induced luminescence and fluorescent probes, applied in the field of detection of heparin, can solve problems such as limited sensitivity and complex synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
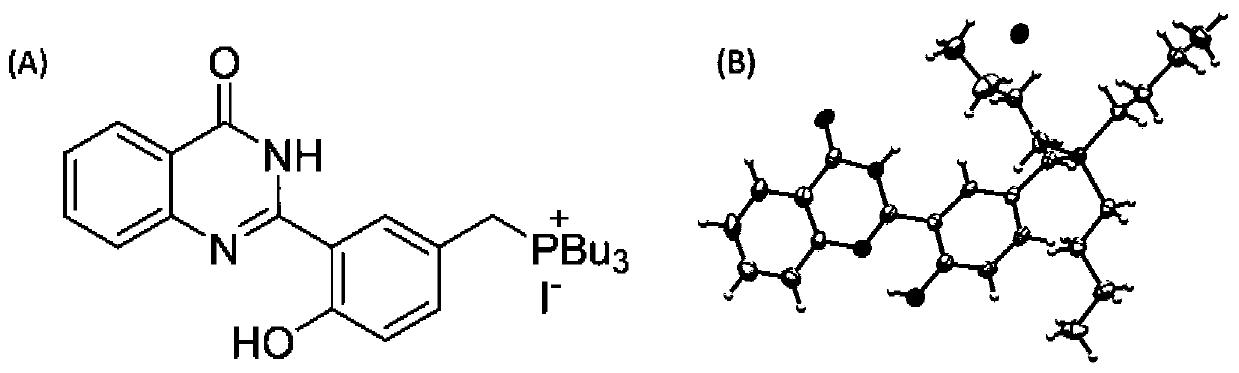
[0052] According to the following synthetic route, the following compounds are specifically synthesized:
[0053]
[0054] (1) Synthesis of tributyl (3-formyl-4-hydroxybenzyl) phosphine (compound 3):
[0055] Add 5-(chloromethyl)-2-hydroxysalicylaldehyde (compound 1) (340 mg, 2 mmol) and tributylphosphine (compound 2) (740 μL, 3 mmol) into THF under reflux and stir for 1 hour (The temperature of reflux is 70 ℃), then continue to react for 10 hours after cooling to room temperature, obtain white solid precipitate, filter precipitate and dry under vacuum (drying temperature is 40 ℃), obtain compound 3, productive rate is 72% (535mg);
[0056] (2) Fluorescent probe tributyl (4-hydroxy-3-(4-oxo-3,4-dihydroquinazolin-2-yl) benzyl) phosphine iodide (HPQ-TBP-I) synthesis:
[0057] Tributyl (3-formyl-4-hydroxybenzyl) phosphine (372 mg, 1 mmol), 2-aminobenzamide (compound 4) (136 mg, 1 mmol) and iodine (254 mg, 1 mmol) were refluxed in ethanol for 6 After the reaction is complet...
Embodiment 2
[0063] Embodiment 2: detection of heparin
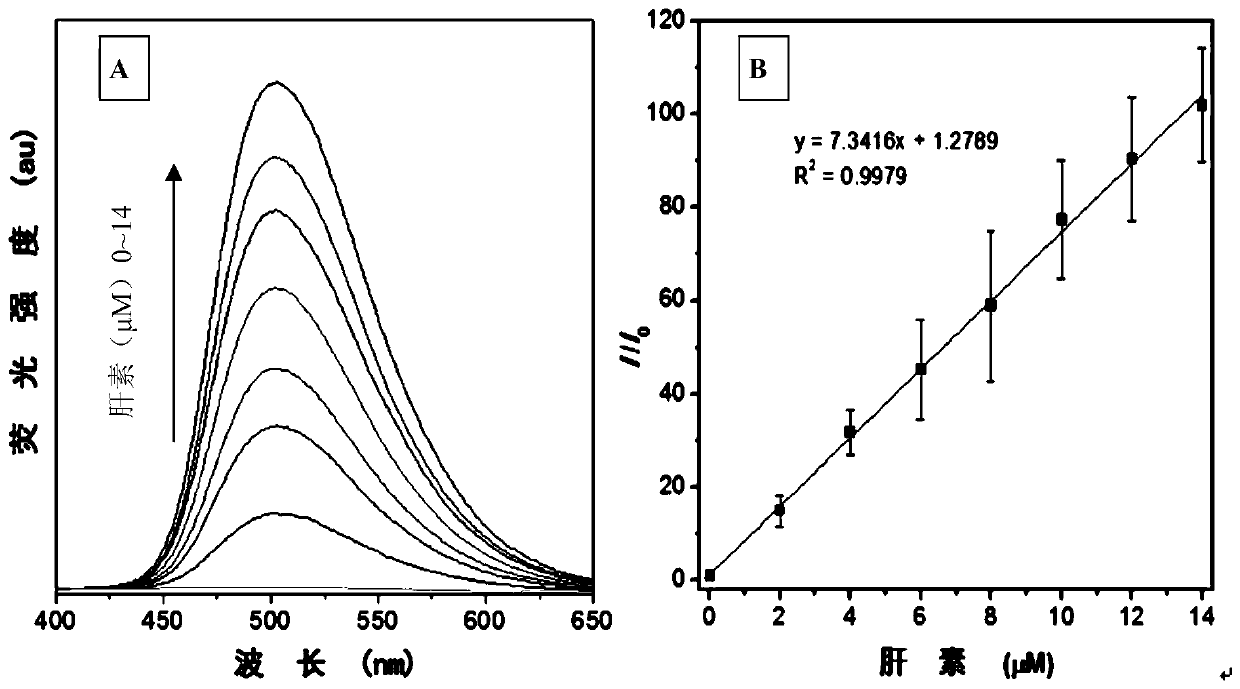
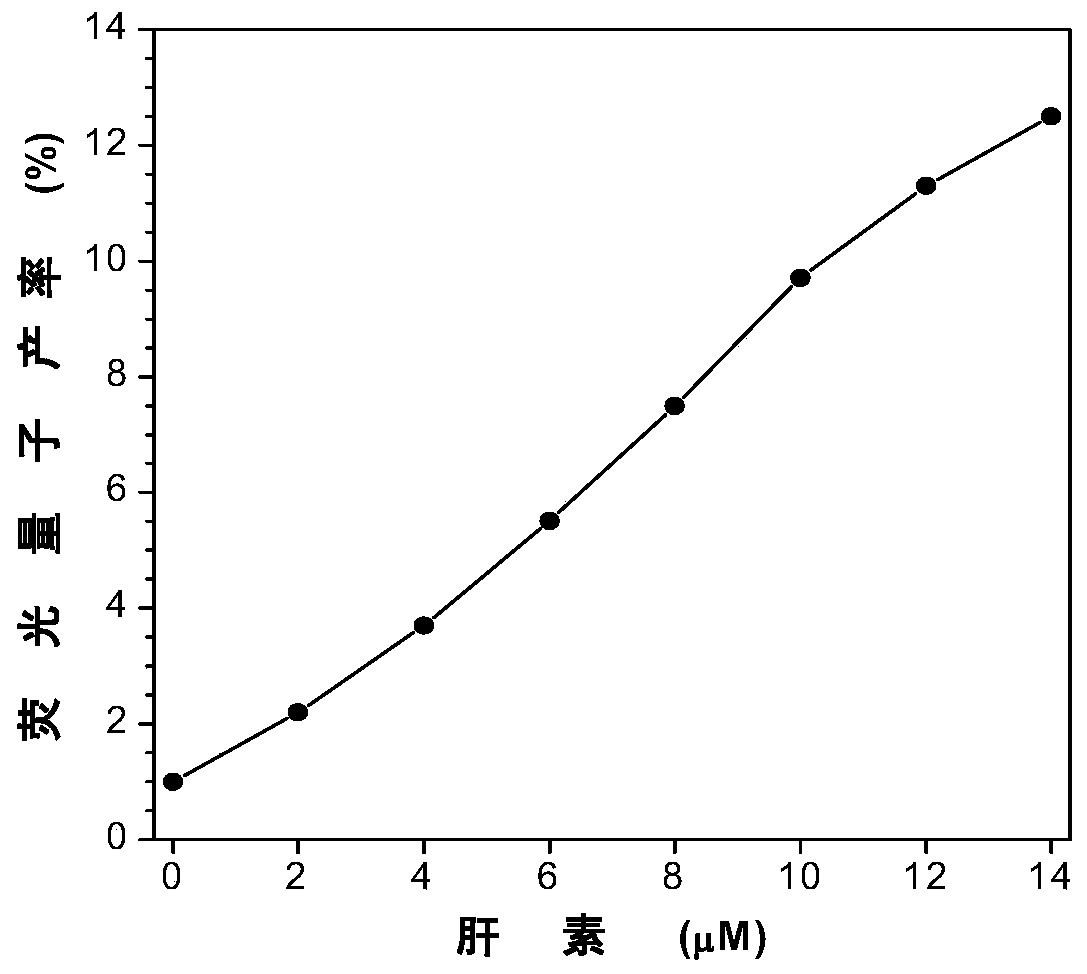
[0064] Add 30 μL of the DMSO (10 mM) mother solution of the fluorescent probe HPQ-TBP-I prepared in Example 1 into 2.970 mL of pure water containing different contents of heparin (Heparin content is 0-14 μM), vortex for 10 seconds, and use Fluorescence was measured with a spectrofluorometer (excitation wavelength 330 nm). Test results such as figure 2 with 3 shown.
[0065] The fluorescent probe HPQ-TBP-I forms a complex with heparin through electrostatic interaction to limit the intramolecular movement and prevent the water in the system from destroying the intramolecular hydrogen bonds of the compound, so as to realize the lighting of heparin.
[0066] figure 2 Middle A is the fluorescence emission spectrum of fluorescent probe HPQ-TBP-I in the heparin of different content (0-14 μ M), figure 2 Middle B is the change curve of the ratio of the fluorescence intensity of the fluorescent probe HPQ-TBP-I at 501nm to the fluoresce...
Embodiment 3
[0068] Embodiment 3: the detection of different water-soluble anionic polymers and ions
[0069] Add 30 μL of the DMSO (10 mM) mother solution of HPQ-TBP-I prepared in Example 1 to 2.970 mL containing different water-soluble anionic polymers (such as heparin (Hep), chondroitin sulfate (ChS), etc.), biomacromolecules (such as pig Hemoglobin (PHB), fetal bovine serum (BSA), hyaluronic acid (HA, etc.) and ions (such as: Na + ,K + ,Mg 2+ , Ca 2+ , Cl - ,PO 4 3– , SO 4 2– ) in pure water, vortexed for 10 s, and measured fluorescence with a spectrofluorometer (excitation wavelength: 330 nm). Test results such as Figure 4 shown.
[0070] Figure 4 It is a histogram of the ratio of the fluorescence intensity of the fluorescent probe HPQ-TBP-I at 501 nm to the fluorescence intensity of HPQ-TBP-I in water in different water-soluble anionic polymers, biomacromolecules and ions. The results show that the probe has a good recognition effect on water-soluble anionic polymers co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com