Gliclazide sustained-release tablet and preparation method thereof
A technology for gliclazide and sustained-release tablets, applied in the field of gliclazide sustained-release tablets and its preparation, can solve problems such as hypoglycemia, achieve the effects of reducing toxic and side effects, improving bioavailability, and stabilizing blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
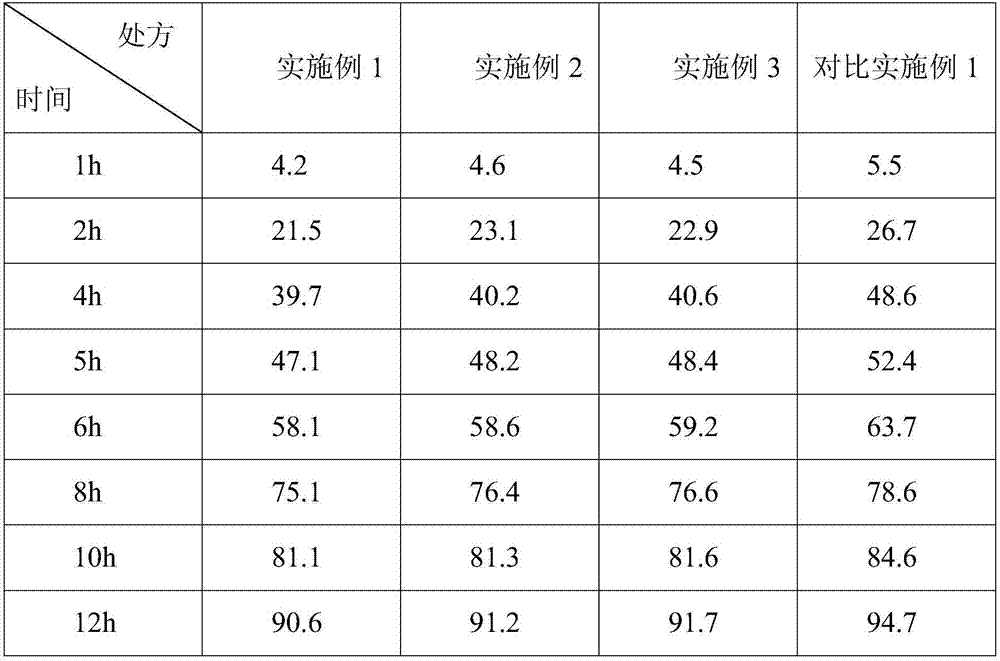
[0019] A gliclazide sustained-release tablet, consisting of a tablet core and a coating layer, calculated in parts by weight, the tablet core includes the following raw and auxiliary materials: 80 parts of gliclazide, 40 parts of lactose, 1 part of magnesium stearate, 4 parts of micropowder silica gel, 42 parts of microcrystalline cellulose and 26 parts of hydroxypropyl methylcellulose; the coating layer is a polymer coating layer containing titanium dioxide and polyethylene glycol, and the coating layer is 2% of the tablet core weight . Gliclazide was ultrafinely pulverized in advance.
[0020] The preparation method comprises the following steps:
[0021] 1) Glicazide is ultrafinely pulverized, and then sieved with magnesium stearate, microcrystalline cellulose, hydroxypropyl methylcellulose, and lactose for subsequent use;
[0022] 2) Add gliclazide, lactose, microcrystalline cellulose and hydroxypropyl methylcellulose into a high-efficiency wet granulator for dry mixing,...
Embodiment 2
[0026] A gliclazide sustained-release tablet, consisting of a tablet core and a coating layer. Calculated in parts by weight, the tablet core includes the following raw and auxiliary materials: 60 parts of gliclazide, 30 parts of lactose, 1 part of magnesium stearate, 1 part of micropowder silica gel, 30 parts of microcrystalline cellulose and 30 parts of hydroxypropyl methylcellulose; the coating layer is a polymer coating layer containing titanium dioxide and polyethylene glycol, and the coating layer is 2.3% of the tablet core weight . Gliclazide was ultrafinely pulverized in advance.
[0027] The preparation method comprises the following steps:
[0028] 1) Glicazide is ultrafinely pulverized, and then sieved with magnesium stearate, microcrystalline cellulose, hydroxypropyl methylcellulose, and lactose for subsequent use;
[0029] 2) Add gliclazide, lactose, microcrystalline cellulose and hydroxypropyl methylcellulose into a high-efficiency wet granulator for dry mixing...
Embodiment 3
[0033] A gliclazide sustained-release tablet, consisting of a tablet core and a coating layer. Calculated in parts by weight, the tablet core includes the following raw and auxiliary materials: 100 parts of gliclazide, 50 parts of lactose, 2 parts of magnesium stearate, 4 parts of micropowder silica gel, 50 parts of microcrystalline cellulose and 10 parts of hydroxypropyl methylcellulose; the coating layer is a polymer coating layer containing titanium dioxide and polyethylene glycol, and the coating layer is 3% of the weight of the tablet core . Gliclazide was ultrafinely pulverized in advance.
[0034] The preparation method comprises the following steps:
[0035] 1) Glicazide is ultrafinely pulverized, and then sieved with magnesium stearate, microcrystalline cellulose, hydroxypropyl methylcellulose, and lactose for subsequent use;
[0036] 2) Add gliclazide, lactose, microcrystalline cellulose and hydroxypropyl methylcellulose into a high-efficiency wet granulator for dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com