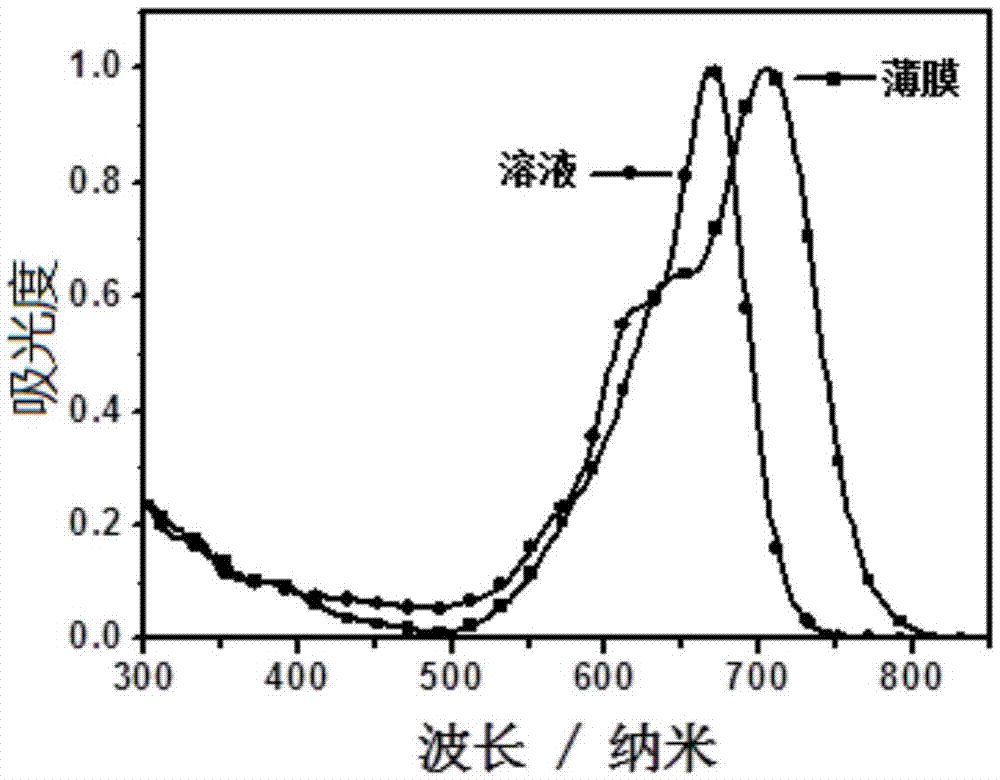
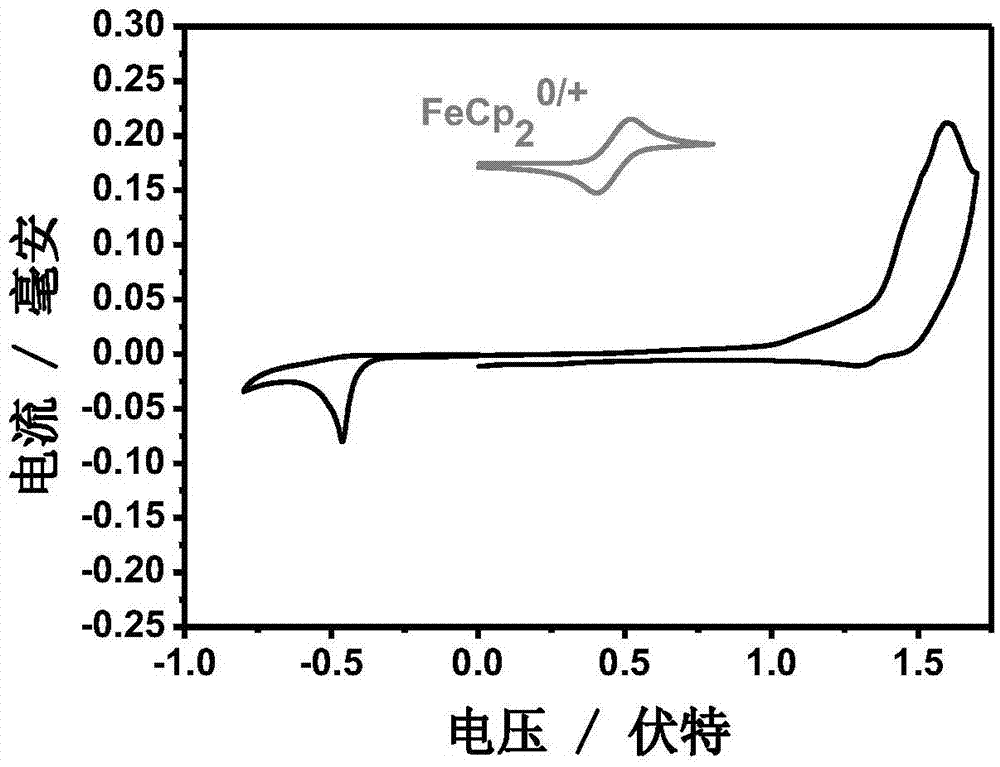
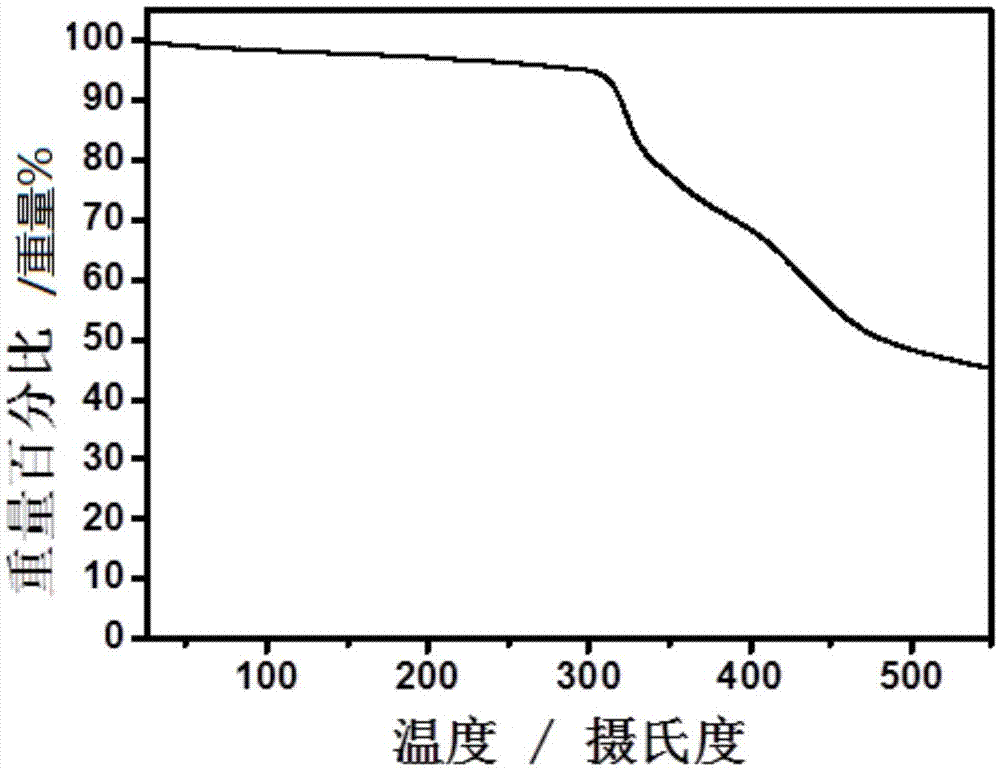
Conjugated macromolecule based on seven parallel condensed nucleus units, preparation method and application thereof in solar cell
A macromolecular and conjugated technology, applied in the field of solar cells, can solve the problems of difficult energy level regulation, difficult purification, weak absorption in the visible light region, etc., and achieve the effect of strong light absorption and high charge transport performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The present invention also provides a method for preparing the above-mentioned conjugated macromolecule based on hepta-fused ring units, wherein the method comprises:
[0081] In the presence of a basic compound and in an organic solvent, the compound represented by the following formula (2) and the compound represented by the formula (B) are dehydrated and condensed to obtain the compound represented by the formula (1); wherein,
[0082] Formula (2)
[0083] Formula (B) is selected from one or more of the following compounds:
[0084]
[0085] Among them, four R 2 independently selected from the group shown;
[0086] two r's 1 independently selected from the group shown;
[0087] Wherein, each Z is independently selected from C or Si;
[0088] Each X and each Y are each independently selected from O, S or Se;
[0089] m is an integer of 1-6;
[0090] n is an integer of 0-6;
[0091] Each R 3 , each R 4 , R 5 , each R 6 and each R 7 Each is indepen...
Embodiment 1
[0179] This example is used to illustrate the conjugated macromolecule based on the hepta-fused ring unit of the present invention and its preparation method.
[0180]
[0181] Among them, C 6 h 13 means n-hexyl
[0182] As shown in the above reaction formula, the compound represented by formula (2-1) (220 mg, 0.2 mmol; purchased from Solon Organic Optoelectronics Technology (Beijing) Co., Ltd.), the compound represented by formula (B-2) (200 mg, 1 mmol; purchased from TCI Company), chloroform (50 mL) and pyridine (10 mL, 12 mmol) were added to the reaction vessel, and argon was passed through for 30 min to remove the air, and then refluxed at 65° C. for 12 h. However, after cooling to room temperature (about 20°C), the reaction product was poured into 200mL of methanol, filtered, and the obtained precipitate was chromatographically used on a silica gel column (using 200-300 mesh silica gel, and the eluent was petroleum ether with a volume ratio of 1:2). / dichloromethane...
Embodiment 2
[0188] This example is used to illustrate the conjugated macromolecule based on the hepta-fused ring unit of the present invention and its preparation method.
[0189]
[0190] Among them, C 6 h 13 means n-hexyl
[0191] As shown in the above reaction formula, the compound shown in formula (3-1) (275mg, 0.2mmol; purchased from Solon Organic Optoelectronics Technology (Beijing) Co., Ltd.), the compound shown in formula (C-1-1) ( 116mg, 0.4mmol; purchased from Suzhou Nakai Technology Co., Ltd.), toluene (50mL) and tetrakis (triphenylphosphine) palladium (10mg, 0.009mmol) were added in the reaction vessel, and argon was passed through for 30min to remove the air, and then Reflux at 110°C for 36h. The solid obtained after spin-drying was chromatographically separated with a silica gel column (using 200-300 mesh silica gel, and the eluent was petroleum ether / dichloromethane with a volume ratio of 1:2) to obtain a dark blue solid (280mg, yield is 95%), which is the conjugated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com