Preparation method of self-support transition metal sulfide foamed electrode for water decomposition
A transition metal, self-supporting technology, applied in the direction of electrodes, electrolysis processes, electrode shapes/types, etc., can solve problems such as hindering electron transfer to current collector electrodes, active material shedding, and limiting overall catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 (Ni / Co-S)
[0013] Nickel foam was washed with dilute hydrochloric acid, ethanol, and ultrapure water as cathode, cobalt rod as anode, cobalt chloride and cobalt sulfate as electroplating solution, and the current density was 2mA cm -2 Electrodeposition was performed for 2 hours; the deposited metal foam was placed in a reaction kettle, and a thiourea solution with a concentration of 0.04M was added for 24 hours of hydrothermal reaction to obtain a self-supporting bifunctional nickel-cobalt sulfide foam electrode.
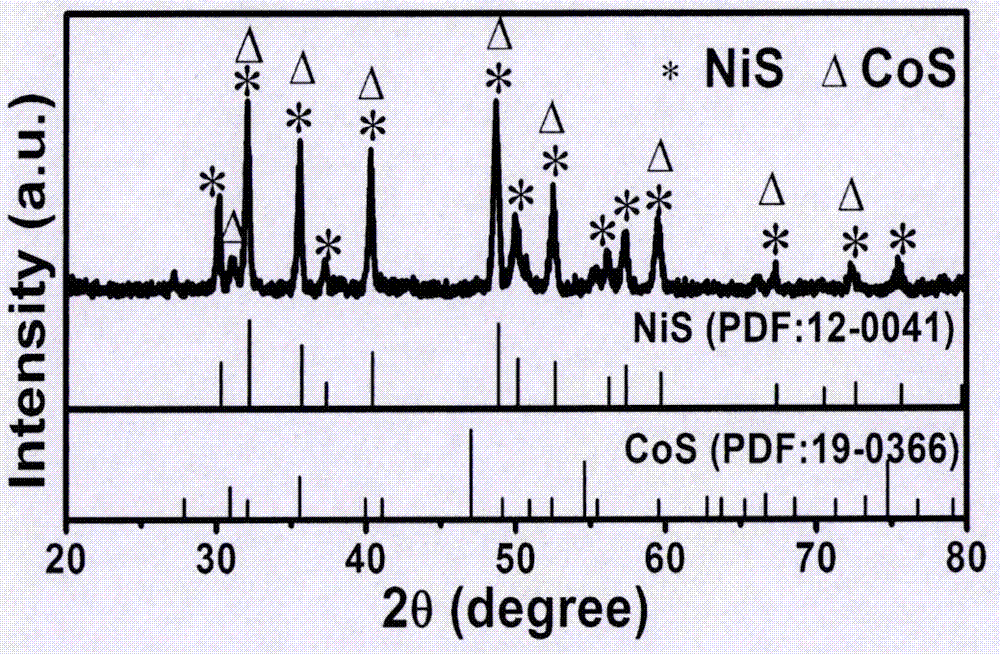
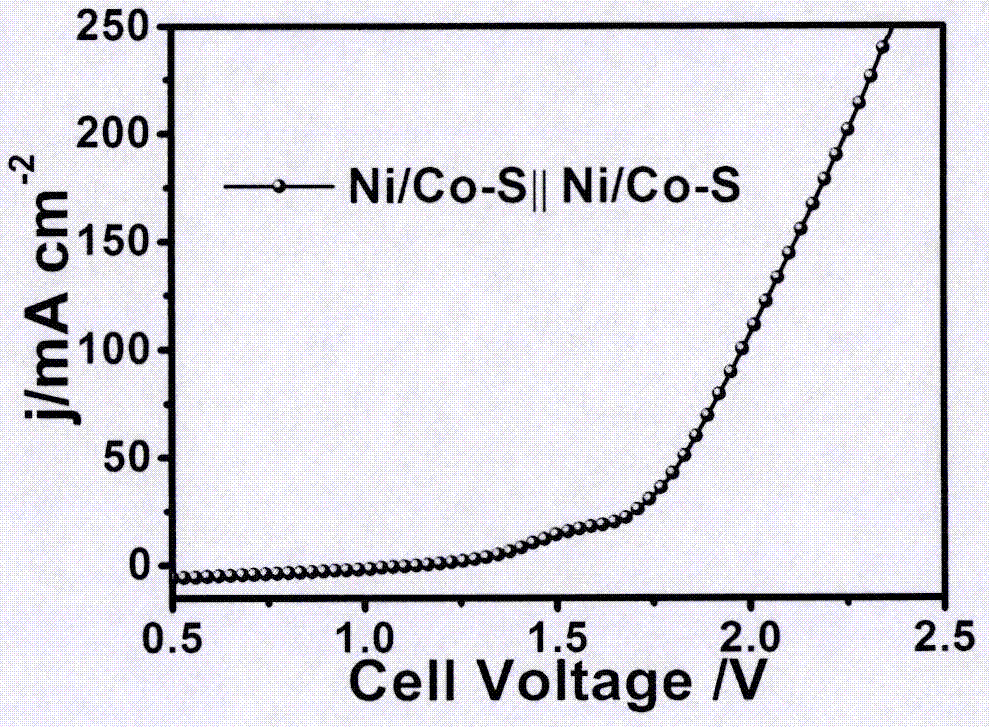
[0014] figure 1 For the X-ray diffraction pattern (XRD) of the nickel-cobalt sulfide foam electrode that embodiment 1 prepares, it can be seen that this electrode contains NiS and CoS two phases, scanning electron microscope (SEM) figure (attachment figure 2 ) It can be seen that rods are closely arranged on the surface of the metal foam. Using the nickel-cobalt sulfide foam electrode as both the cathode and the anode, electrochemical voltammetr...
Embodiment 2
[0015] Example 2 (Ni / Mo-S)
[0016] The nickel foam was washed with dilute hydrochloric acid, ethanol, and ultrapure water as the cathode, the molybdenum rod as the anode, and the sodium molybdate as the electroplating solution with a current density of 2mA cm -2 Electrodeposition was carried out for 2 hours; the deposited metal foam was placed in a reaction kettle, and a thiourea solution with a concentration of 0.04M was added for 24 hours of hydrothermal reaction to obtain a self-supporting bifunctional nickel-molybdenum sulfide foam electrode. attached Figure 5 The scanning electron microscope image of the electrode shows that the network structure is wound on the surface of the foam matrix, which increases the specific surface area of the electrode.
Embodiment 3
[0017] Example 3 (Ni / NiMo-S)
[0018] The nickel foam was washed with dilute hydrochloric acid, ethanol, and ultrapure water as the cathode, the carbon rod was used as the anode, and sodium molybdate, nickel sulfate, and ammonium citrate were used as the electroplating solution, and the current density was 2mA cm -2 Electrodeposition was carried out for 1 hour; the deposited metal foam was placed in a reaction kettle, and a thiourea solution with a concentration of 0.05M was added for 12 hours of hydrothermal reaction to obtain a self-supporting bifunctional nickel-molybdenum sulfide foam electrode.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com