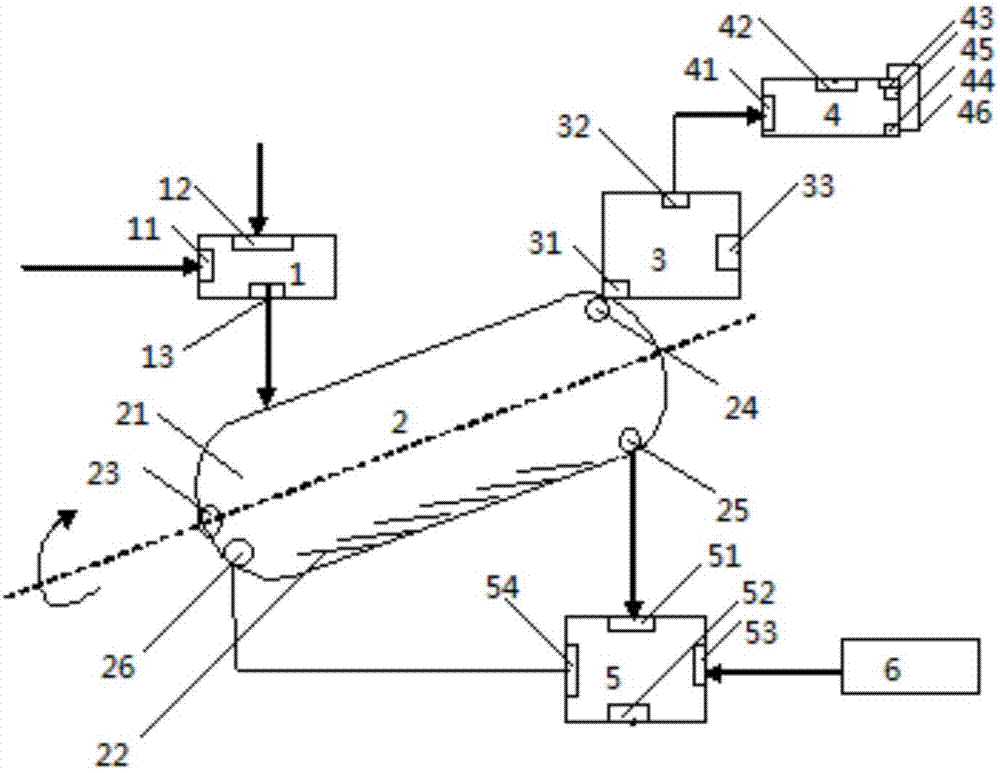
Method for production of potassium sulfate from industrial by-product ammonium sulfate
A technology of ammonium sulfate and by-products, applied in the direction of sulfate/bisulfate preparation, etc., can solve the problems of ammonium sulfate difficult to handle, chemical fertilizer potassium sulfate production process complex, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] 2000kg of ammonium sulfate (a by-product of the preparation of methyl methacrylate, wherein the water content is 10%, containing 0.8% of organic impurities) and 2054kg of potassium chloride (purity 98.0%) are stirred and mixed, mixed for 2.0h, and put into a high-temperature reactor , at 400°C, roasted at 60rpm (stirring speed) for 2.0h, at the same time, feed 20°C air into the high temperature reactor, the air flow rate is 6m 3 / h, the solid product after roasting is recovered after being cooled by a potassium sulfate cooler, and the gaseous product after roasting is fixed through a gas trap, wherein the potassium sulfate product is calculated by the weight of potassium element, and the recovery rate of potassium is 98.5%. The purity of the obtained potassium sulfate is 98.7%, which meets agricultural needs; the ammonium chloride product is based on the weight of nitrogen, and the recovery rate of ammonium is 98%, and the purity of ammonium chloride is 98.0%. Compared ...
Embodiment 2
[0110] 2000kg of ammonium sulfate (a by-product of the preparation of methyl methacrylate, wherein the water content is 12%, containing 1.2% of organic impurities) and 1999kg of potassium chloride (purity 98.0%) are stirred and mixed, mixed for 1.5h, and put into a high-temperature reactor , at 420°C, roasting at 40rpm (stirring speed) for 3.0h, at the same time, feed 20°C air into the high temperature reactor, the air flow rate is 10m 3 / h, the solid product after roasting is recovered after being cooled by a potassium sulfate cooler, and the gaseous product after roasting is fixed through a gas trap, wherein the potassium sulfate product is calculated by the weight of potassium element, and the recovery rate of potassium is 98.8%. The purity of the obtained potassium sulfate is 98.5%, which meets agricultural needs; the ammonium chloride product is based on the weight of nitrogen, and the recovery rate of ammonium is 97.8%, and the purity of ammonium chloride is 98.4%. Calcul...
Embodiment 3
[0113] 2000kg of ammonium sulfate (a by-product of the preparation of methyl methacrylate, wherein the water content is 5%, containing 0.5% of organic impurities) and 2176kg of potassium chloride (purity 98.0%) are stirred and mixed, mixed for 1.5h, and put into a high-temperature reactor , at 300°C, roasting at 60rpm (stirring speed) for 2.5h, at the same time, feed 20°C air into the high temperature reactor, the air flow rate is 2m 3 / h, the solid product after roasting is recovered after being cooled by a potassium sulfate cooler, and the gaseous product after roasting is fixed through a gas trap, wherein the potassium sulfate product is calculated by the weight of potassium element, and the recovery rate of potassium is 98.9%. The purity of the obtained potassium sulfate is 98.0%, which meets agricultural needs; the ammonium chloride product is based on the weight of nitrogen, and the recovery rate of ammonium is 95.8%, and the purity of ammonium chloride is 98.2%. Compare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com