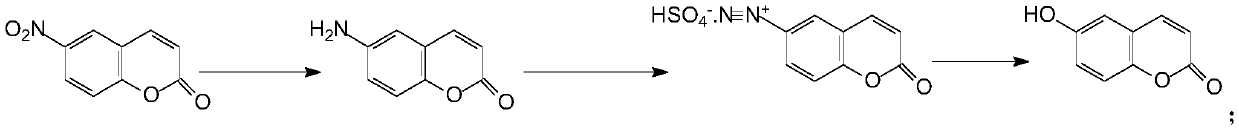
The preparation method of 6-hydroxycoumarin
A technology for hydroxycoumarin and aminocoumarin, which is applied in the field of preparation of 6-hydroxycoumarin, can solve the problems of expensive reagents, complicated preparation process, troublesome purification, etc., and achieves reduction of production cost, simple preparation process, and source of Wide range of convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a clean 1L four-neck flask, add 100g 6-nitrocoumarin, 10.5g ferric chloride hexahydrate, 26.25g medicinal activated carbon, 500ml alcohol / N,N-dimethylformamide mixed solvent ( The ratio is 2:8v / v), stirred and mixed, heated to about 80°C, and kept for 30 minutes; then at 80°C, slowly added 81.8g of 80% hydrazine hydrate dropwise, and the dropping time was 30 minutes. At an internal temperature of 90-100°C, the stirring reaction was continued for 2 hours. After the reaction is completed, lower the temperature to 80°C, filter while it is hot, and remove the activated carbon. The filtrate is a light yellow to orange-red clear liquid; the solution is cooled to room temperature, and slowly poured into water with 3 times the amount of the solution, and a light yellow to light yellow crystal is precipitated. Solid, continue cooling at about 10°C for 2 hours, crystallization is complete; filter and dry to obtain 75.86g of 6-aminocoumarin in the form of light yellow crystalli...
Embodiment 2
[0028] In a clean 1L four-neck flask, add 100g 6-nitrocoumarin, 14.06g ferric chloride hexahydrate, 35.15g medicinal activated carbon, 700ml alcohol / N,N-dimethylformamide mixed solvent ( The ratio is 2:8v / v), stir and mix, heat to about 80°C, keep for 30 minutes; then slowly add 154.5g of 80% hydrazine hydrate dropwise at 80°C, dropwise for 30 minutes, after dropping, heat to At an internal temperature of 95-100°C, the stirring reaction was continued for 2 hours. After the reaction is completed, lower the temperature to 80°C, filter while it is hot, and remove the activated carbon. The filtrate is a light yellow to orange-red clear liquid; the solution is cooled to room temperature, and slowly poured into water with 3 times the amount of the solution, and a light yellow to light yellow crystal is precipitated. Solid, continue cooling at about 10°C for 2 hours, crystallization is complete; filter and dry to obtain 70.2g of 6-aminocoumarin in the form of light yellow crystalline...
Embodiment 3
[0029] Embodiment 3 (laboratory scale-up test):
[0030] In a clean 10L four-neck flask, add 800g 6-nitrocoumarin, 84g ferric chloride hexahydrate, 210.0g medicinal activated carbon, 4L alcohol / N,N-dimethylformamide mixed solvent ( The ratio is 2:8v / v), stirred and mixed, heated to about 80°C, and kept for 30 minutes; then at 80°C, slowly added 654.4g of 80% hydrazine hydrate dropwise, and the dropping time was 45-60 minutes, and the dropwise was completed. Heat to an internal temperature of 90-100°C, and continue to stir and react for 2 hours. After the reaction is completed, lower the temperature to 80°C, filter while it is hot, and remove the activated carbon. The filtrate is a light yellow to orange-red clear liquid; the solution is cooled to room temperature, and slowly poured into water with 3 times the amount of the solution, and a light yellow to light yellow crystal is precipitated. Solid, continue to cool at about 10°C for 2 hours, crystallization is complete; filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com