Chelating agent having multidentate aminocarboxylic-type dimer, preparation method and application thereof, and separation medium therewith
A technology for separating medium and dimer, which is applied in the direction of solid adsorbent liquid separation, ion exchange of chelate, separation method, etc., can solve the problems of only suitable synthesis method, uncommon, complicated steps, etc., and achieve experimental operation. The effect of convenience and safety, short steps and simple reaction device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
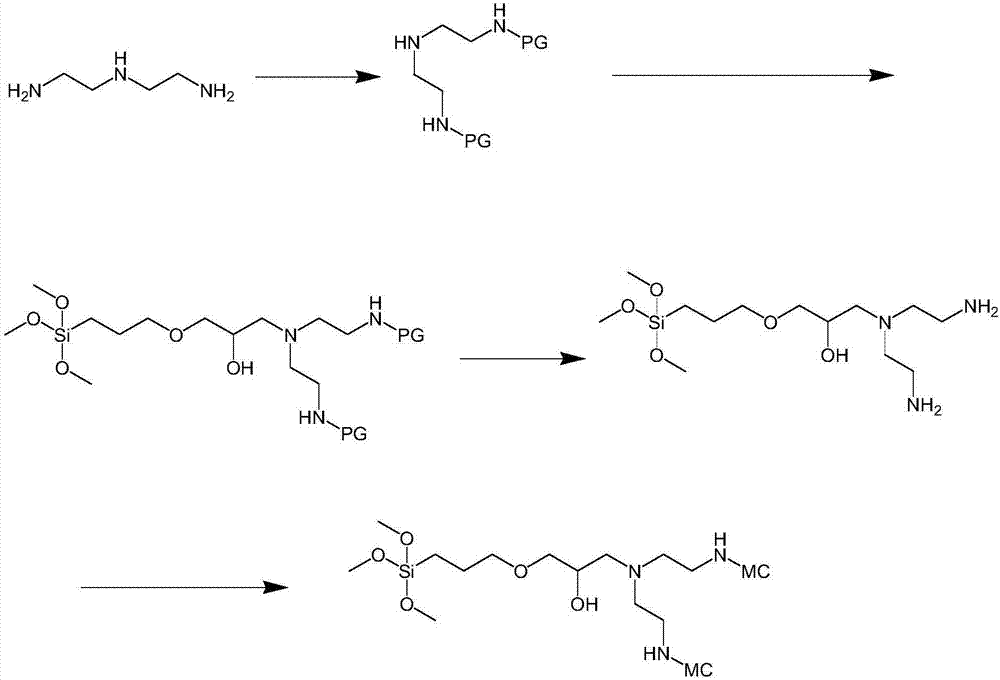
[0082] A kind of preparation method of described polydentate aminocarboxyl dimer chelating agent, it comprises the steps:
[0083] 1) Diethylenetriamine reacts with an amino protection reagent to protect the primary amino group;
[0084] 2) Diethylenetriamine after primary amino group protection reacts with γ-glycidyl etheroxypropyltrimethoxysilane;
[0085] 3) The product obtained in step 2) is subjected to a deprotection reaction to remove the protecting group of the primary amine;
[0086] 4) The primary amino group of the product obtained in step 3) reacts with the aminocarboxyl ester to form an amide bond.
[0087] Preferably, in step 1), the molar ratio of the diethylenetriamine to the amino-protecting reagent is 1:1.9 to 2.1; more preferably, the molar ratio of the diethylenetriamine to the amino-protecting reagent is 1: 2.
[0088] Preferably, in step 1), the protecting group for protecting the primary amine includes one of tert-butoxycarbonyl, methoxycarbonyl, etho...
Embodiment 1
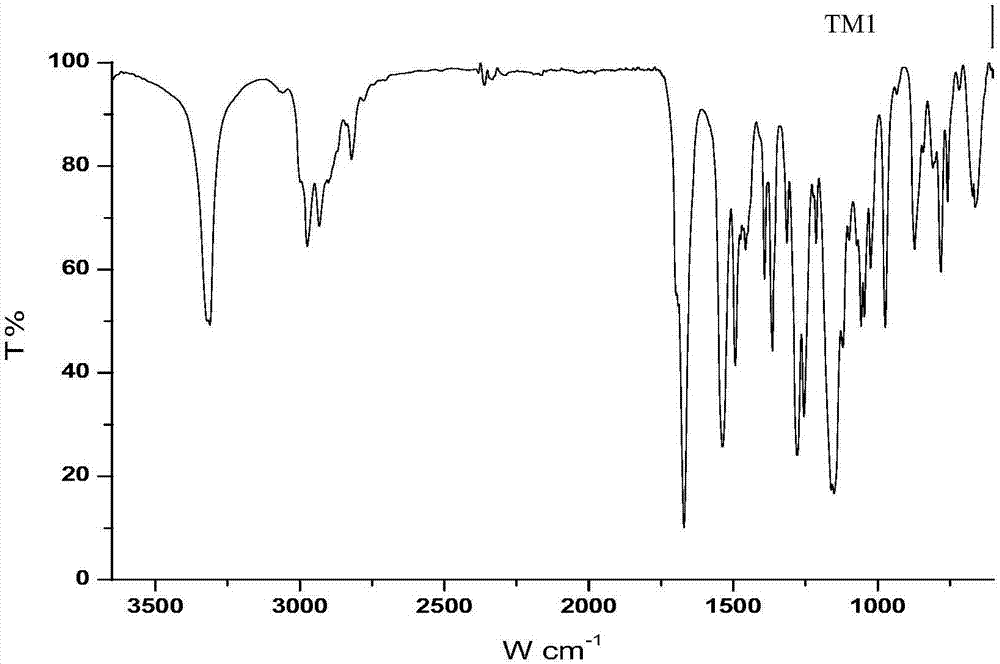
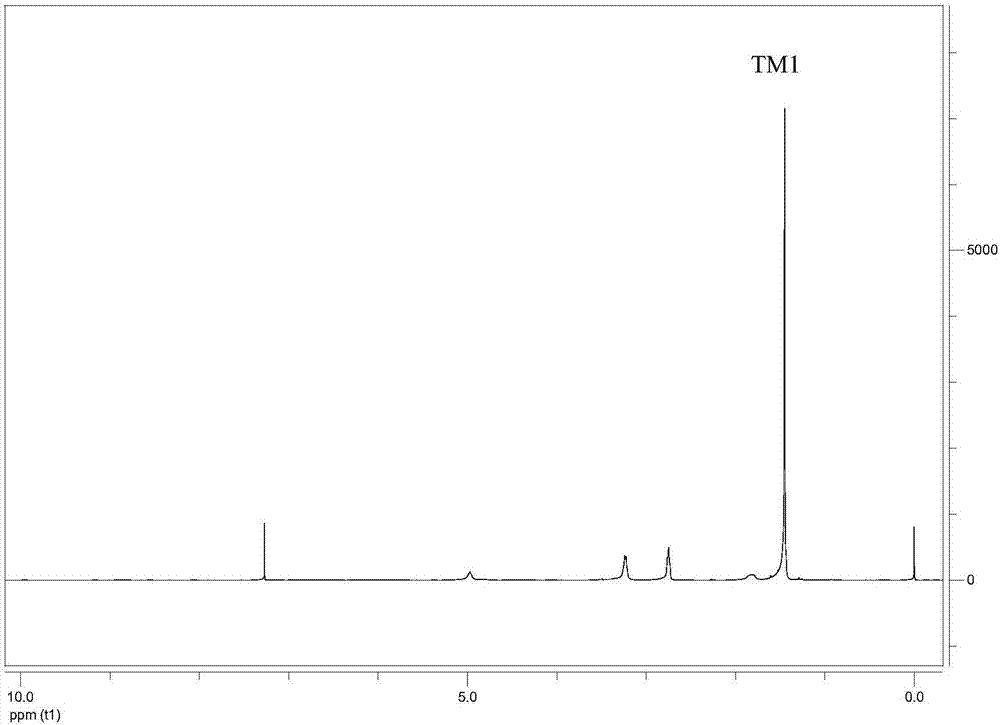
[0099] Preparation of TM1
[0100]
[0101] In a 150mL flask, dissolve 1.10mL (10.30mmol) of diethylenetriamine (DETA) in 20mL of tetrahydrofuran, then add 4.28mL (30.90mmol) of triethylamine, and stir in a low-temperature reaction bath at 0°C for 30min; weigh 5.2131 g (20.60mmol) 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile (Boc-on), and dissolved in 60mL tetrahydrofuran, placed in a constant pressure dropping funnel, added dropwise to the above in solution. After 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile was completely dropped into the flask, the reaction was continued at 0° C. for 4 h in a low-temperature reaction bath, and then left at room temperature for 3 h.
[0102] 1.1 TLC analysis
[0103] This step reaction carries out thin-layer chromatography to each reactant, developing agent is dichloromethane (DCM):methanol (MeOH)=9:1, the R of raw material DETA f The value is 0.16, because there are three amino groups in the molecular structure of DETA...
Embodiment 2
[0110] Under alkaline conditions, TM1 reacts with γ-GLDP to generate TM2:
[0111]
[0112] Measure 20mL of ethanol (EtOH) and put it in a beaker, then weigh 1.3g of sodium metal, cut it into small pieces and place it in it, and transfer it to a 150mL flask after the sodium metal has completely reacted; weigh 2.9160g (9.61mmol) of TM1 Add to the flask and stir to dissolve completely; accurately measure 1.5mL (6.79mmol) of γ-glycidyl etheroxypropyltrimethoxysilane (γ-GLDP), dissolve it in 3mL of ethanol, and add it dropwise under rapid stirring in the flask. The flask was placed in a water bath at 80°C and refluxed for 12h under rapid stirring.
[0113] After the reaction finished, the ethanol in the flask was completely steamed out with a rotary evaporator; 50 mL of distilled water was added to the flask to hydrolyze EtONa; the solution was rotary evaporated again at a temperature of 55° C., and the ethanol in the aqueous solution was steamed; 6 mol / L HCl was added to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com