Drug compound containing tuberculosis allergen CE
A technology for allergens and compositions, which can be used in compound screening/testing, pharmaceutical formulations, preparations for in vivo tests, etc., and can solve problems such as poor tuberculosis specificity, incomplete PPD components, and inaccurate tuberculosis infection rates. , to achieve the effect of strong immunogenicity, improved sensitivity, and improved microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
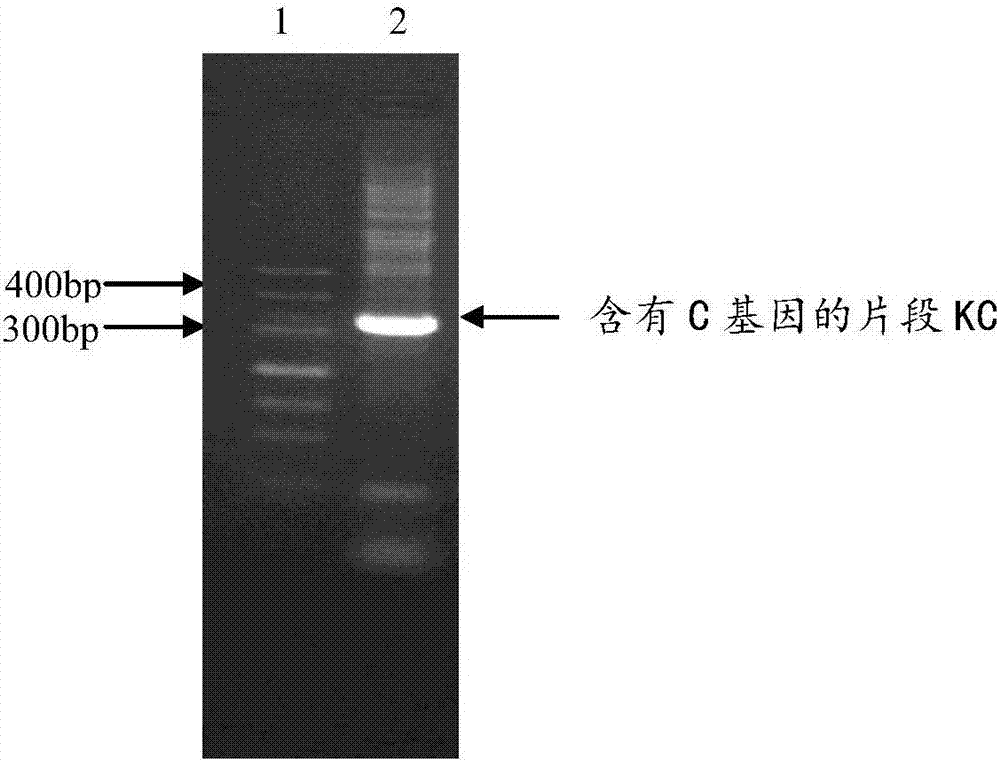
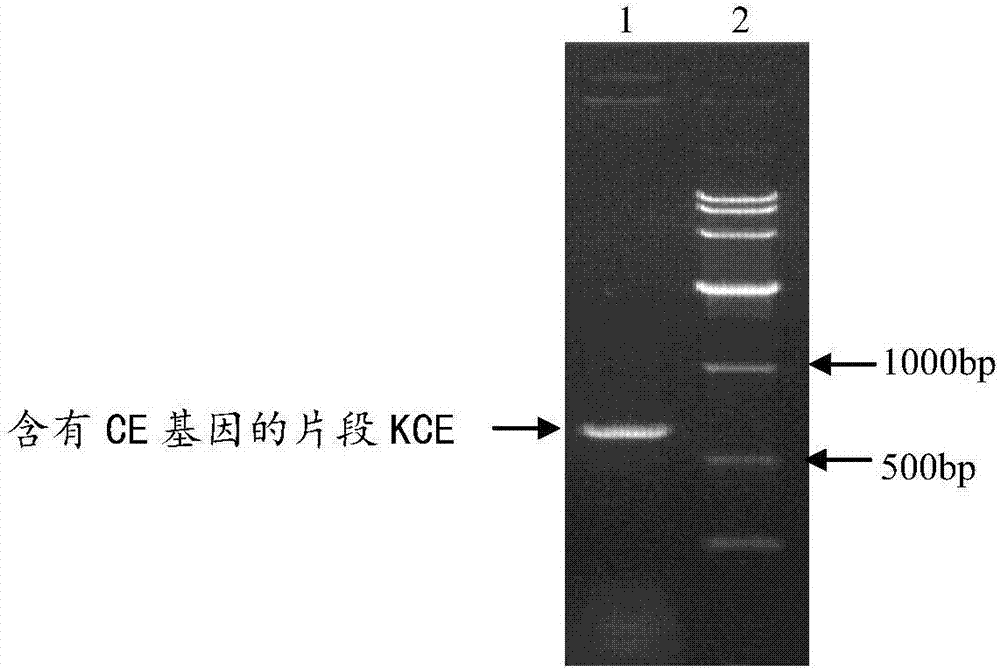
[0084] Embodiment 1, the cloning of the coding gene of recombinant fusion protein CE and the expression and purification of recombinant fusion protein CE
[0085] 1. Design of fusion of two protein epitopes
[0086] Analyze the gene sequence and protein structure of Mycobacterium tuberculosis CFP10 and ESAT6, determine the fusion region, combination and sequence of the epitopes of the two proteins, and select the codon with high frequency as the best codon according to the codon usage frequency of Escherichia coli codons or preferred codons, remove some rare or low-utilization codons, and optimize by replacing the original codons with synonymous codons, so that the designed gene can be expressed at a high level in E. coli, increase protein production, and enable protein production more efficient and economical.
[0087] The sequence shown in SEQ ID No.1 was designed. In SEQ ID No.1, the 1st-6th position is restriction endonuclease Nde I digestion recognition site 5'-CATATG-3...
Embodiment 2
[0253] Example 2: Formulation pH Study
[0254] Solution pH is one of the important parameters affecting the physical and chemical stability of biopharmaceuticals. pH can adjust the charge distribution on the surface of the protein, thereby affecting the intramolecular and intermolecular forces of biopharmaceuticals, and directly affects the solubility of the protein. May have lower colloidal stability (Colloidal stability).
[0255] 1. Prescription design
[0256] The optimal pH is determined by investigating the influence of different pH values on the stability of the preparation solution of this product. The pH range was designed to be 2.6-8.0 (20mM citrate-phosphate buffer system), and a prescription was designed every 0.2 pH, and a total of 28 prescriptions with different pH were prepared.
[0257] 2. Prescription configuration
[0258] 20mM pH 2.6 citric acid / citrate buffer and 20mM pH 8.0 phosphate buffer were respectively prepared, and then mixed in different pro...
Embodiment 3
[0274] Example 3: Screening of Prescription Structure Protectors and Formulation Confirmation Research
[0275] The commonly used structural protective agents for protein drugs include polyols (such as mannitol, glycerol), sugars (such as sucrose and trehalose), and amino acids (such as glycine, arginine). The protection mechanism of the above-mentioned structure protectors is called "preferential exclusion", that is, in aqueous solution, the protein preferentially excludes the protector molecules from the protein surface, and the addition of these protectors will increase the chemical potential energy of the protein. Due to the increased surface area of protein denaturation and unfolding, and the increase in interaction with excipients, the chemical potential energy increases, and the activation energy for protein conformational denaturation increases, so that the balance between the natural conformation and the denatured conformation of the protein shifts to the natural con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com