Method for recycling hydrogen isotopes from hydrogen isotope lithium compound
A hydrogen isotope and lithium compound technology, which is applied in the recovery of hydrogen isotopes and the field of hydrogen isotope recovery, can solve the problems of short time, high operating temperature, and low operating temperature, and achieve the effect of short time, low operating temperature and improved safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
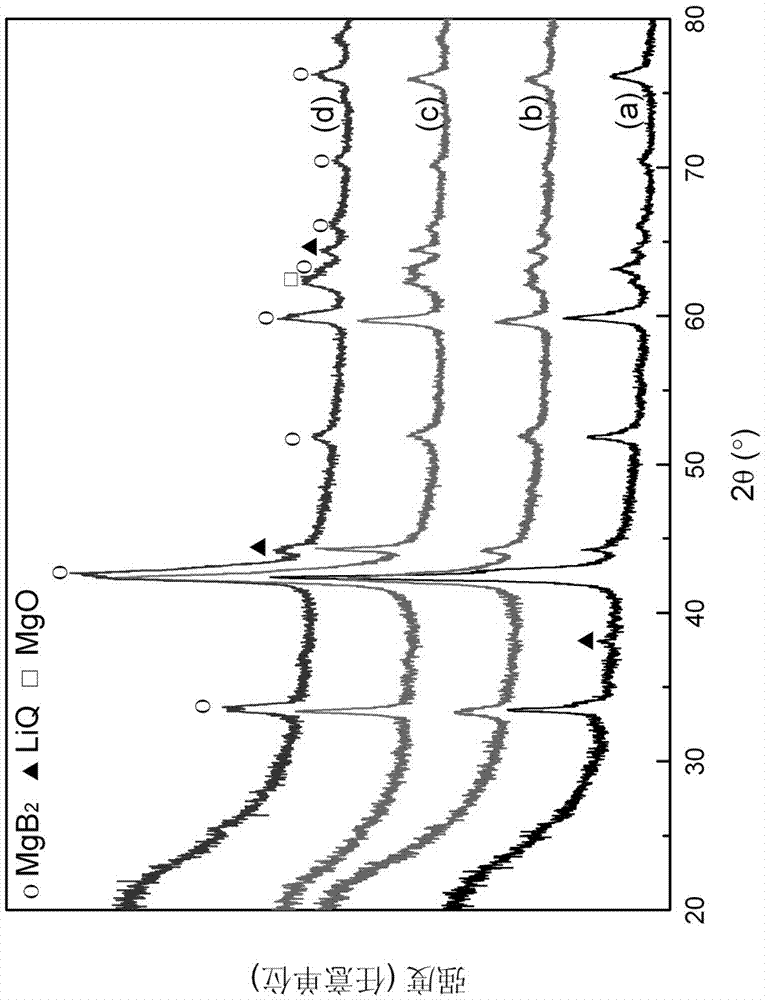
[0041] In a glove box filled with argon, weigh the two raw material powders of lithium deuteride and magnesium diboride at a molar ratio of 1:1, pour them into a stainless steel ball mill jar with a volume of 100 ml, and grind them under an argon atmosphere. Next, use a planetary ball mill to mill for 2 h, and carry out mechanical mixing treatment to obtain the first mixture, and its XRD pattern is as follows: figure 1 (a) shown.
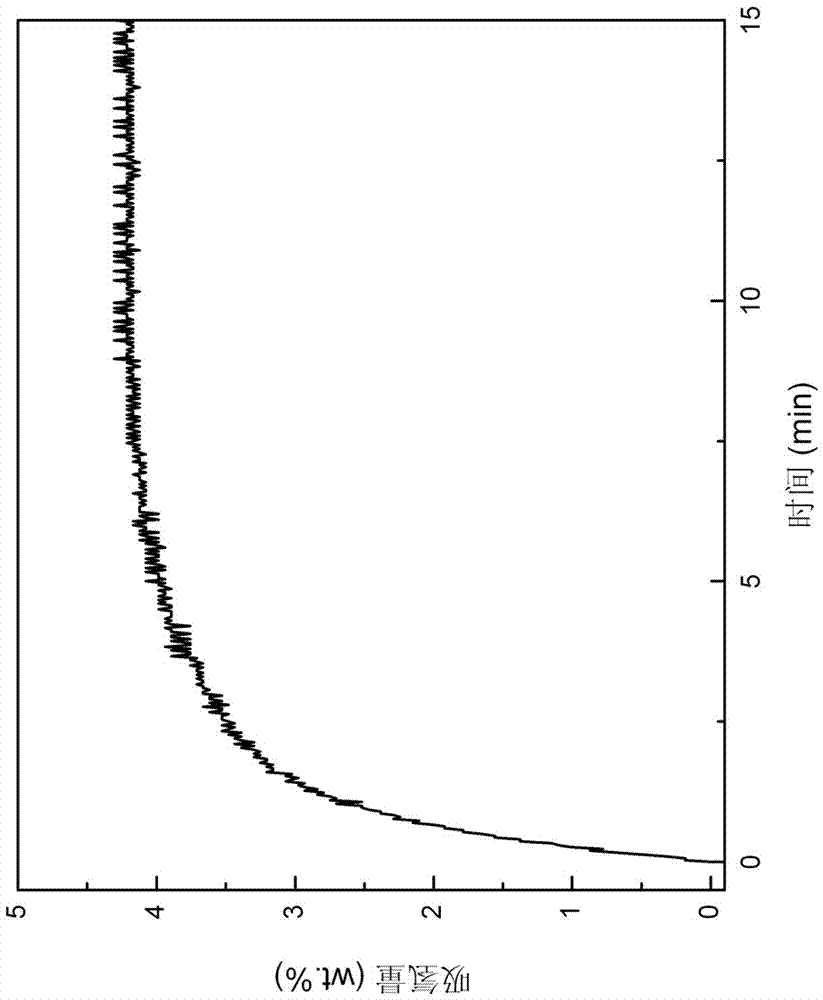
[0042] Put the first mixture obtained from the ball mill into the reactor, vacuumize it, put the reactor into a heating furnace, and heat the reactor to 400°C; then, quickly fill the reactor with 99.99% purity Hydrogen to 6 MPa, carry out the hydrogen absorption reaction for 15 minutes, the hydrogen absorption reaction curve is as follows figure 2 shown. From figure 2 It can be seen from the figure that the hydrogen absorption is basically completed after the reaction takes about 10 min. Afterwards, the temperature of the reactor was kept at 4...
Embodiment 2
[0044]In a glove box filled with argon, weigh the two raw material powders of lithium deuteride and magnesium diboride at a molar ratio of 2:1, pour them into a stainless steel ball mill jar with a volume of 100 ml, and grind them under an argon atmosphere. Next, use a planetary ball mill to mill for 1 h, and carry out mechanical mixing treatment to obtain the first mixture.
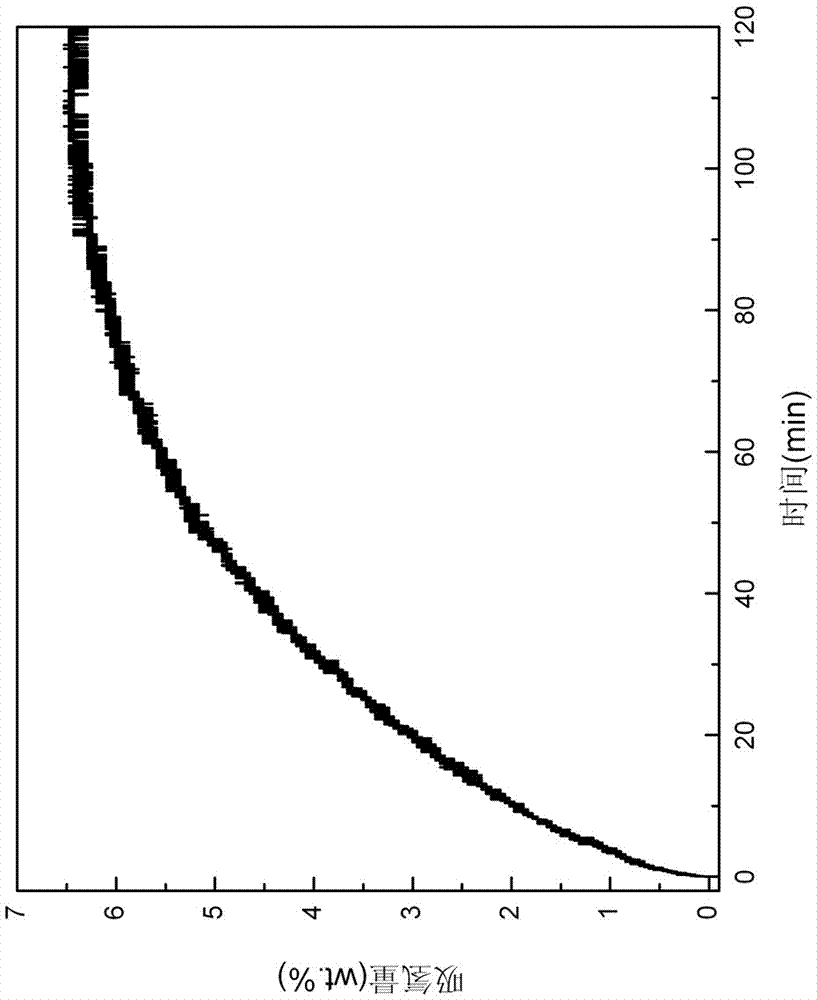
[0045] Put the first ball-milled mixture into the reactor, vacuumize it, put the reactor into the heating furnace, and heat the reactor to 350°C; then, quickly fill the reactor with 99.99% purity Hydrogen to 5MPa, carry out the hydrogen absorption reaction for 120 min, the hydrogen absorption reaction curve is as follows image 3 shown. From image 3 It can be seen from the figure that the hydrogen absorption is basically completed after the reaction takes about 90 min. XRD after hydrogen absorption as Figure 4 As shown, it can be found that after absorbing hydrogen, they are all converted into LiBQ...
Embodiment 3
[0047] In a glove box filled with argon gas, two kinds of raw material powders of lithium deuteride and magnesium diboride were weighed at a molar ratio of 2:1, and were ground with a mortar for 10 min under an argon atmosphere, and mixed to obtain the first a mixture.
[0048] Put the first ball-milled mixture into the reactor, vacuumize it, put the reactor into a heating furnace, and heat the reactor to 250°C; then, quickly fill the reactor with hydrogen with a purity of 99.99% to 7 MPa for hydrogen absorption reaction. After the hydrogen absorption is completed, the temperature of the reactor is cooled to room temperature, the hydrogen pressure is reduced to 0 bar, and then the reactor is heated at a heating rate of 5°C / min to cause the dehydrogenation reaction of the hydrogen absorption product, and the deuterium can be released from the dehydrogenation reaction. released during the process, the hydrogen desorption curve is as follows Figure 6 As shown, it can be found ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com