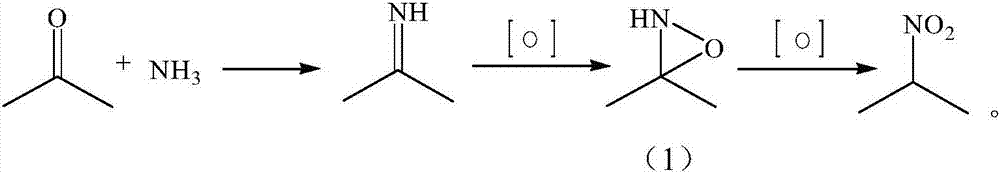
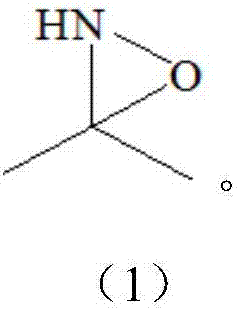
Synthesizing method of 2-nitropropane
A technology for nitropropane and a synthesis method, which is applied in the field of preparation of substituted saturated hydrocarbons, can solve the problems of excessive acetone oxime yield, difficulty in balancing the overall production load, and the like, and achieves the effects of simple post-processing, convenient operation, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 6 g (0.1 mol) of acetamide, 58 g (1 moL) of acetone, and 150 g of water into the reaction vessel, and set the temperature of the water bath to 35°C. First slowly pass in about 20-25g of ammonia gas until the temperature in the reaction vessel no longer changes and there is a slight positive pressure in the vessel, raise the temperature and keep the temperature at 40±2°C, add 136g (1.1moL) of 27.5% hydrogen peroxide evenly within 2 hours, drop During the addition, 10 g of ammonia gas was passed into the container 4 times. After the hydrogen peroxide drops, keep warm for 1 hour, and take a sample to detect that the GC content of acetone is <1%, otherwise a small amount of hydrogen peroxide needs to be added.
[0028] Raise the temperature to 75±2°C in the reaction vessel, add 124g (1.0moL) of 27.5% hydrogen peroxide dropwise evenly within 2h, keep it warm for 1h after dropping, drop the temperature to 30-40°C, and add about 15-20g of 31% hydrochloric acid dropwise to ...
Embodiment 2
[0032] The catalyst in the embodiment 1 is replaced by the ammonium acetate catalyst 6g, and the others are the same as in the embodiment 1. After the hydrogen peroxide is dripped for the first time and the heat preservation is finished, the GC content of acetone is 6% in a sample, and 25 g of hydrogen peroxide needs to be added. 67 g of the final product were obtained.
[0033] As detected by GC, the 2-nitropropane content in the final product was 99.0% (wt%), and the total molar yield of 2-nitropropane in terms of acetone was 76%.
Embodiment 3
[0035] Change the amount of acetamide catalyst in Example 1 to 3g, and the others are the same as in Example 1. After the hydrogen peroxide is dripped for the first time and the heat preservation finishes, the GC content of acetone is sampled at 4%, and 20g of hydrogen peroxide needs to be added. 69 g of the final product were obtained.
[0036] As detected by GC, the 2-nitropropane content in the final product was 99.1% (wt%), and the total molar yield of 2-nitropropane in terms of acetone was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com