Self-assembly polyimide porous material, preparation method thereof and application thereof in lithium sulfur battery
A technology of polyimide and porous materials, which is applied in the fields of polyimide particles, porous carbon precursors, and positive electrode carbon materials. It can solve the problems of positive electrode structure collapse, capacity drop, and poor conductors, and achieve mild reaction conditions Reduce contact resistance and facilitate charging and discharging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] At room temperature, add 0.200g of 4,4-diaminodiphenyl ether (ODA) and PVB of equal mass to 6.5ml of DMF solution, stir vigorously to disperse evenly, add 0.266g of 1,4,5,8- Naphthalene tetracarboxylic anhydride, stirred at room temperature for 10 hours.
[0031] Transfer the above solution into a polytetrafluoroethylene liner, put the reaction kettle in an oven, react at 180°C for 10h, cool to room temperature, filter, wash, and dry to obtain porous polyamic acid particles.
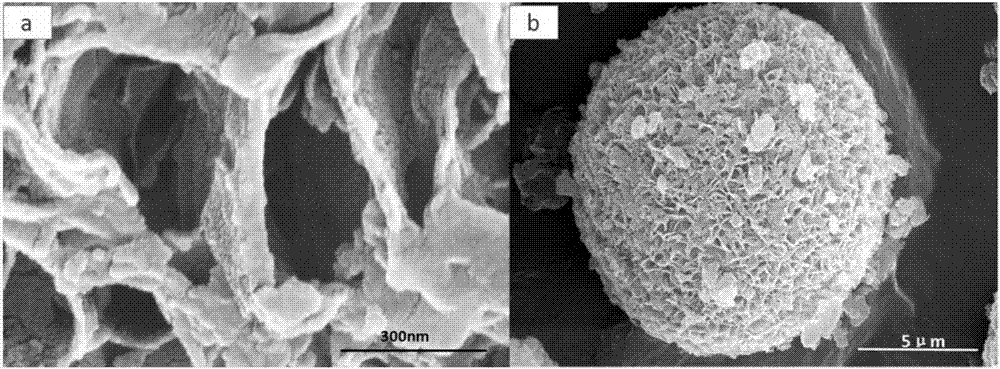
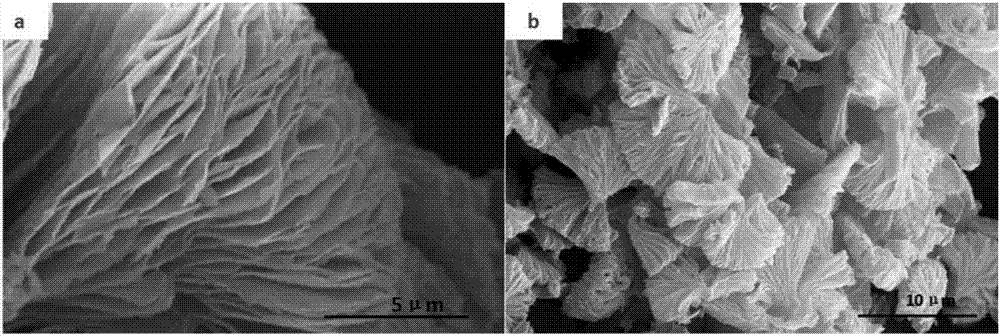
[0032] The obtained polyimide acid particles were placed in a tube furnace under N 2 Under the atmosphere, the temperature was raised to 400°C at 3°C / min and maintained for 1h to obtain polyimide particles; the temperature was continued to be raised to 900°C at 3°C / min and maintained for 1h to obtain porous carbon spheres.
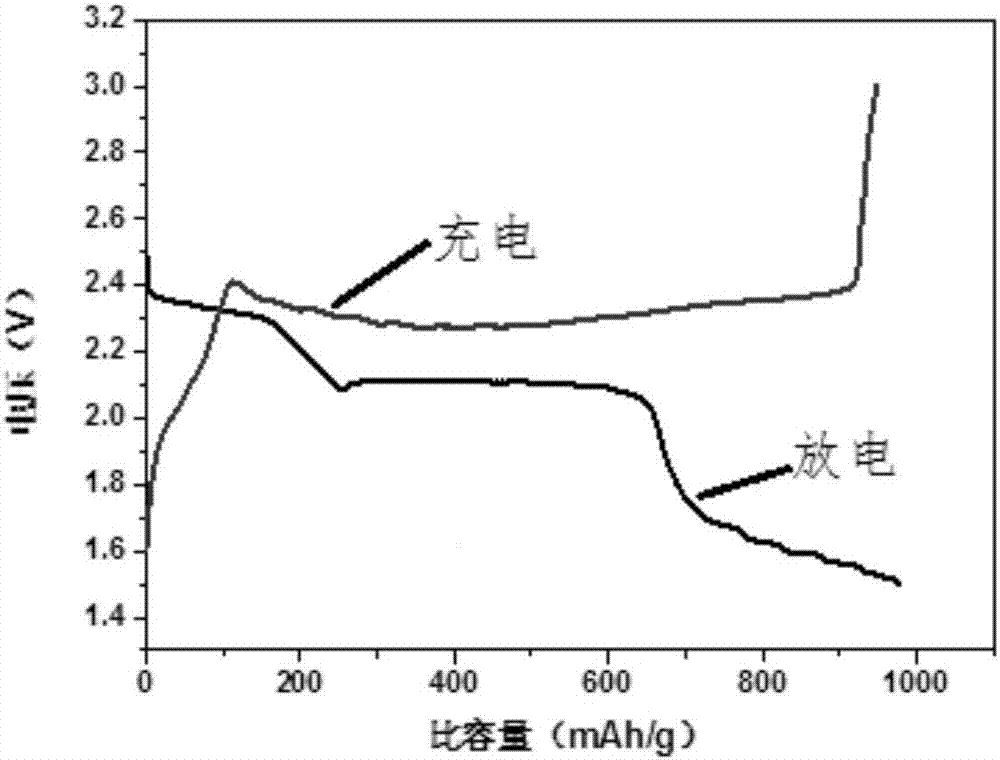
[0033] Dissolve 0.19g of elemental sulfur in 40ml of toluene, weigh 0.08g of the above-mentioned carbon material, mix the sulfur toluene solution with the carbon material, st...
Embodiment 2
[0040] At room temperature, add 0.4234g of 4,4-diaminodiphenyl ether (ODA) and equal mass of PVB to 13.2ml of DMF solution, stir vigorously to disperse evenly, add 0.6460g of 1,4,5,8-naphthalene tetrachloride in batches Formic anhydride, stirred at room temperature for 9 hours.
[0041] Transfer the above solution into a polytetrafluoroethylene liner, place the reaction kettle in an oven, react at 180°C for 10h, cool to room temperature, filter, wash, and dry to obtain porous polyamic acid particles.
[0042] Place the obtained polyimide acid particles in a tube furnace, raise the temperature to 350°C at 3°C / min under N2 atmosphere, and keep it for 1h to obtain polyimide particles; continue to raise the temperature to 800°C at 3°C / min , maintained for 1h, to obtain porous carbon spheres.
[0043] Dissolve 0.3g of elemental sulfur in 60ml of toluene, weigh 0.3g of the above-mentioned carbon material, mix the toluene solution of sulfur with the carbon material, stir at room tempe...
Embodiment 3
[0050] At room temperature, add 0.445g of 4,4-diaminodiphenyl ether (ODA) and equal mass of PVB to 13ml of 1-methyl-2-pyrrolidone (NMP) solution, stir vigorously to disperse evenly, add 0.530g1 in batches ,4,5,8-Naphthalene tetracarboxylic anhydride was added to the above solution, stirred vigorously to disperse evenly, and stirred at room temperature for 10 hours.
[0051] Transfer the above solution into a polytetrafluoroethylene liner, place the reaction kettle in an oven, react at 180°C for 10h, cool to room temperature, filter, wash, and dry to obtain porous polyamic acid particles.
[0052] Place the obtained polyimide acid particles in a tube furnace, raise the temperature to 350°C at 3°C / min under N2 atmosphere, and maintain it for 1h to obtain polyimide particles; continue to raise the temperature to 900°C at 3°C / min , maintained for 3h, to obtain porous carbon spheres.
[0053] Dissolve 0.19g of elemental sulfur in 40ml of toluene, weigh 0.08g of the above-mentioned...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com