Preparation method for heterojunction photocatalyst and application
A photocatalyst and heterojunction technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of unsatisfactory photocatalytic degradation effect of organic pollutants and low degradation efficiency of organic pollutants , low photocatalytic degradation performance, etc., to achieve the effects of excellent photocatalytic degradation activity, effective photocatalytic degradation, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
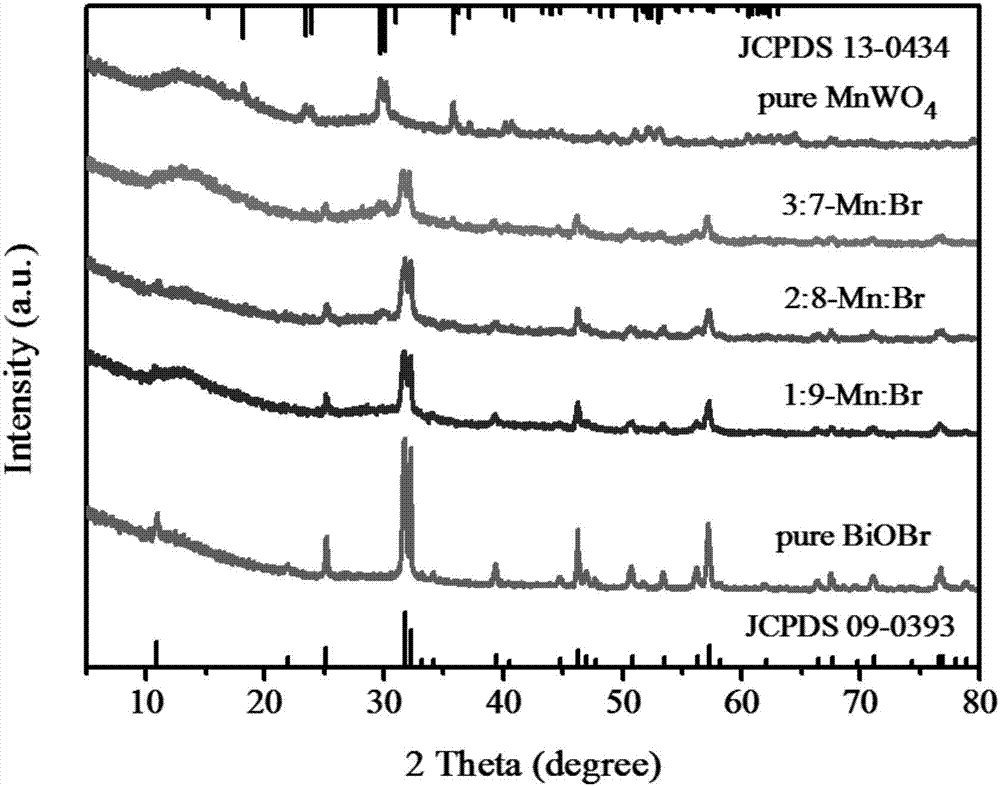
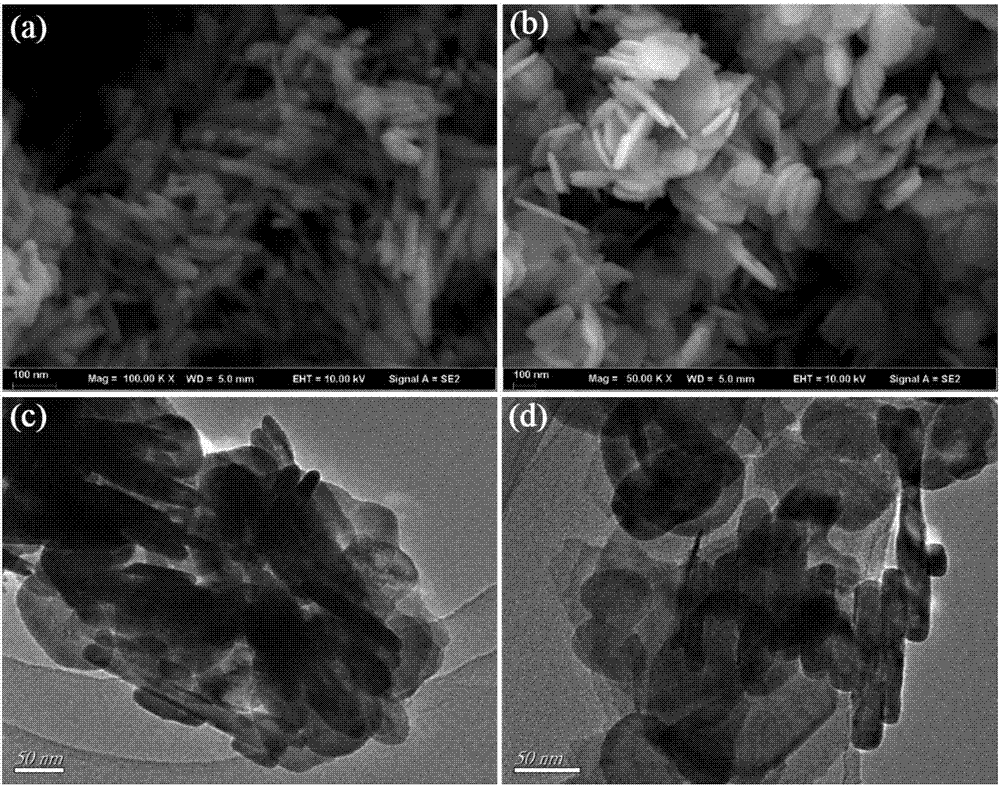
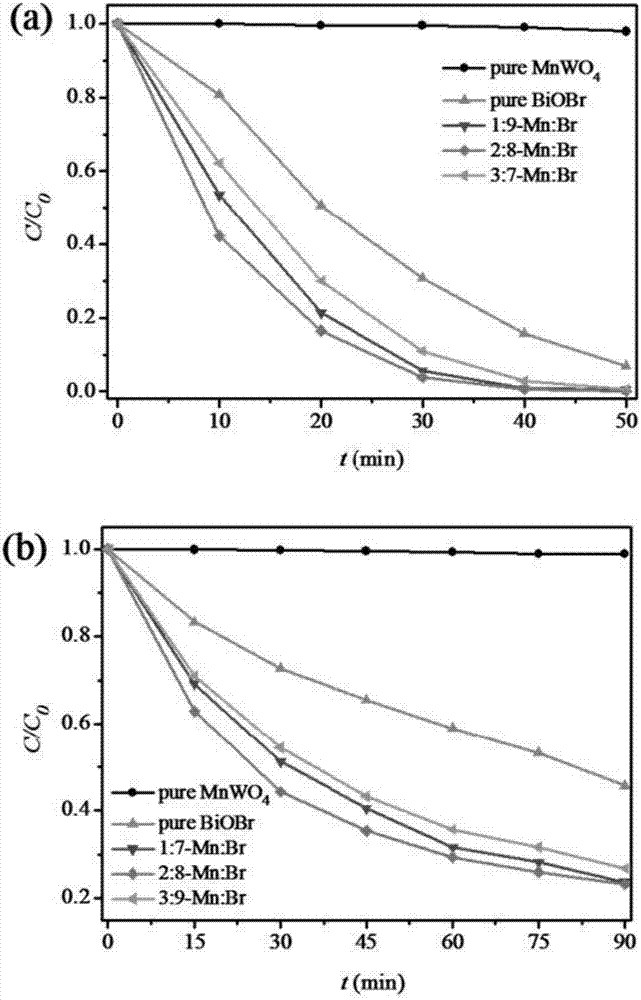
[0029] Step (1): Weigh 1mmol manganous chloride (0.198g) and 1mmol sodium tungstate (0.330g) and dissolve them in 30mL deionized water, ultrasonically dissolve to obtain a suspension, and then adjust the pH with 1mmol / L sodium hydroxide solution value to 9, then transferred to a 50mL hydrothermal reaction kettle, hydrothermally reacted at 180°C for 24h, finally washed with water, filtered, and dried to obtain manganese tungstate A.
[0030] Step (2): Weigh 1mmol of bismuth nitrate (0.485g) and dissolve it in 20mL of ethylene glycol, and ultrasonically dissolve to obtain a white suspension; weigh 1mmol of potassium bromide (0.119g) and dissolve it in 10mL of deionized water, and ultrasonically disperse to obtain a transparent solution, and then the potassium bromide solution was added dropwise to the above-mentioned bismuth nitrate suspension, stirred at room temperature for 30min after the dropwise addition, and then transferred to a 50mL hydrothermal reaction kettle, hydrother...
Embodiment 2
[0033]Step (1): Weigh 1mmol manganous chloride (0.198g) and 1mmol sodium tungstate (0.330g) and dissolve them in 30mL deionized water, ultrasonically dissolve to obtain a suspension, and then adjust the pH with 1mmol / L sodium hydroxide solution value to 9, then transferred to a 50mL hydrothermal reaction kettle, hydrothermally reacted at 180°C for 24h, finally washed with water, filtered, and dried to obtain manganese tungstate A.
[0034] Step (2): Weigh 1mmol of bismuth nitrate (0.485g) and dissolve it in 20mL of ethylene glycol, and ultrasonically dissolve to obtain a white suspension; weigh 1mmol of potassium bromide (0.119g) and dissolve it in 10mL of deionized water, and ultrasonically disperse to obtain a transparent solution, and then the potassium bromide solution was added dropwise to the above-mentioned bismuth nitrate suspension, stirred at room temperature for 30min after the dropwise addition, and then transferred to a 50mL hydrothermal reaction kettle, hydrotherm...
Embodiment 3
[0037] Step (1): Weigh 1mmol manganous chloride (0.198g) and 1mmol sodium tungstate (0.330g) and dissolve them in 30mL deionized water, ultrasonically dissolve to obtain a suspension, and then adjust the pH with 1mmol / L sodium hydroxide solution value to 9, then transferred to a 50mL hydrothermal reaction kettle, hydrothermally reacted at 180°C for 24h, finally washed with water, filtered, and dried to obtain manganese tungstate A.
[0038] Step (2): Weigh 1mmol of bismuth nitrate (0.485g) and dissolve it in 20mL of ethylene glycol, and ultrasonically dissolve to obtain a white suspension; weigh 1mmol of potassium bromide (0.119g) and dissolve it in 10mL of deionized water, and ultrasonically disperse to obtain a transparent solution, and then the potassium bromide solution was added dropwise to the above-mentioned bismuth nitrate suspension, stirred at room temperature for 30min after the dropwise addition, and then transferred to a 50mL hydrothermal reaction kettle, hydrother...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com