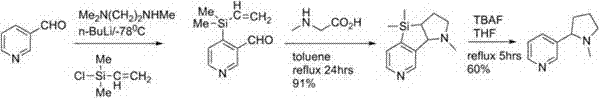
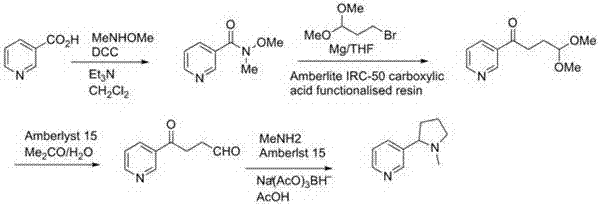
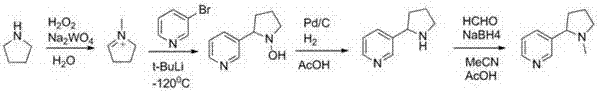
Preparation method of artificially synthesized raceme nicotine
A technology of artificial synthesis and racemate, applied in the direction of organic chemistry, can solve the problems of many steps, increased cost, and inability to scale production, etc., and achieve the effect of mild reaction conditions, simple process, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of racemate nicotine
[0035] 4-Methylamino-1-(3-pyridine)-butanone hydrochloride (13.8g, 0.055mol), dissolved in 160mL water, adjusted with 5mol / L potassium hydroxide or sodium hydroxide at -5°C Weakly alkaline to pH 8, stirred and reacted for 5 hours, concentrated, and the concentrate was refined with a mixture of water and methanol to obtain an intermediate. The intermediate was dissolved in a mixture of 180mL (how much) water and ethanol, and sodium borohydride (3.8g) was added at -5°C, heated to 15°C and stirred for 2 hours, extracted with ethyl acetate, dried and concentrated to give a light yellow oil Add 110mL of water to the crude product, distill it off directly, extract the water with 270mL of n-hexane or tetrahydrofuran, dry and concentrate to obtain 7.9g of oil, with a yield of 72%. HPLC purity 99.2%. (In the following examples, the amount of the above red words must be supplemented)
Embodiment 2
[0036] Embodiment 2: the synthesis of racemate nicotine
[0037] 4-Methylamino-1-(3-pyridine)-butanone hydrochloride (25.1g, 0.1mol), dissolved in 150mL of water and methanol, adjusted to pH 8.5 with sodium carbonate or potassium carbonate at 0°C, stirred for reaction After 3 hours, concentrate and refine the concentrate with a mixture of water and chloroform to obtain an intermediate. The intermediate was dissolved in 160mL of methanol, and lithium tetrahydrohydride (3.7g, 0.1mol) was added at 0°C, heated to 25°C, stirred and reacted for 2 hours, extracted with dichloromethane, dried and concentrated to obtain a crude light yellow oil, added water 210mL, directly distilled out, water was extracted with 290mL petroleum ether or diethyl ether, dried and concentrated to obtain 12.5g of oil. HPLC purity 99.5%.
Embodiment 3
[0038] Embodiment 3: the synthesis of racemate nicotine
[0039] 4-Methylamino-1-(3-pyridine)-butanone hydrochloride (25.1g, 0.1mol), dissolved in 165 mL of water and ethanol, adjusted to pH 7.5 with sodium bicarbonate or potassium bicarbonate at 5°C, stirred After reacting for 2 hours, concentrate and refine the concentrate with a mixture of water and dichloromethane to obtain an intermediate. Dissolve the intermediate in 180mL of propanol or isopropanol, add aluminum trichloride (13.3g, 0.1mol) at 5°C, raise the temperature to 25°C, stir for 2 hours, add 200mL of distilled water, steam distillation, distilled The aqueous phase was extracted with 350 mL of ethyl acetate, and the ethyl acetate was concentrated to dryness to obtain 12.0 g of light yellow oil. HPLC purity was 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com