Method for detecting acyl chloride in medicine or synthesized intermediate thereof by derivatization HPLC-DAD method
A technology of derivatization and derivatization reaction, which is used in the determination of acid chloride by derivatization HPLC-DAD method and the determination of acid chloride in drugs or their synthetic intermediates by derivatization HPLC-DAD method, which can solve the problems of strong ultraviolet absorption and insufficient specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] 1.3. Solution preparation
[0048] Stock solutions of various acid chlorides: Take about 10mg of each acid chloride, accurately weigh it, put it in a 10mL measuring bottle, dilute with acetonitrile to the mark, and shake well. Each stock solution has good stability within 8 hours at room temperature.
[0049] Aniline, 2-nitroaniline (2-NA), 4-nitroaniline (4-NA), phenylhydrazine, 2-nitrophenylhydrazine (2-NPH) and 4-nitrophenylhydrazine hydrochloride (4 -NPH·HCl) test solution: Take about 20 mg of each reagent, weigh it accurately, place it in a 10 mL measuring bottle, dilute to the mark with pure acetonitrile, and shake well.
[0050] 1.4. Derivatization experimental conditions
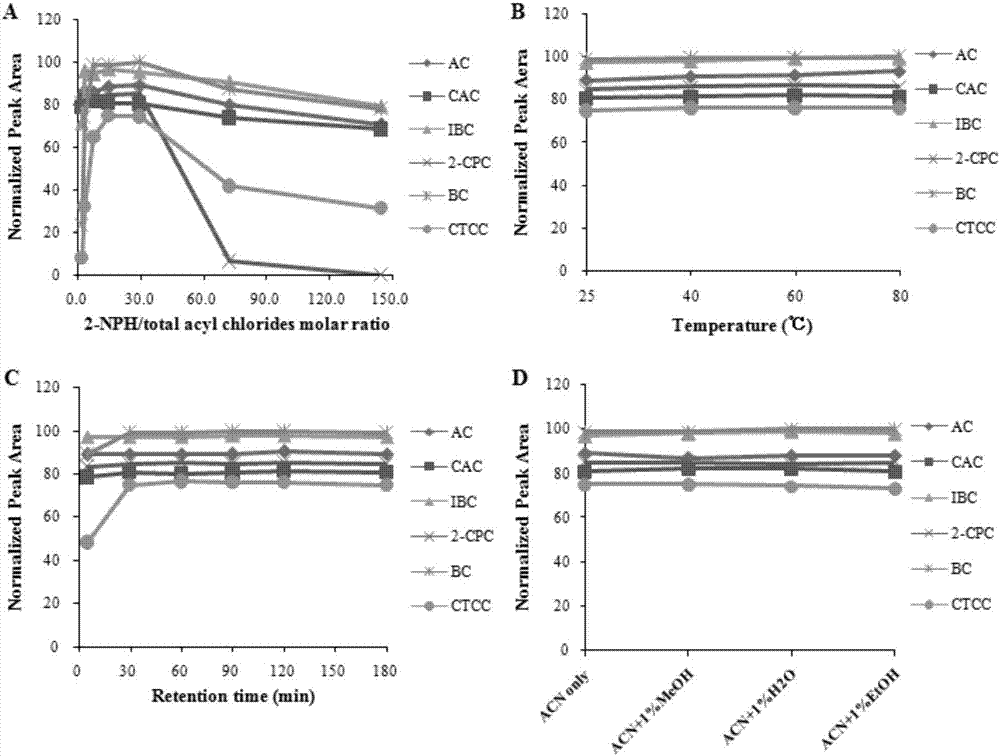
[0051] Precisely pipette 100 μL of the acid chloride mother solution, put it in a 10 mL volumetric flask, add 500 μL of derivatization reagent and acetonitrile to dilute to the mark, and shake well. After reacting at room temperature for 1 h, 20 μL was taken and injected directly.
Embodiment 1
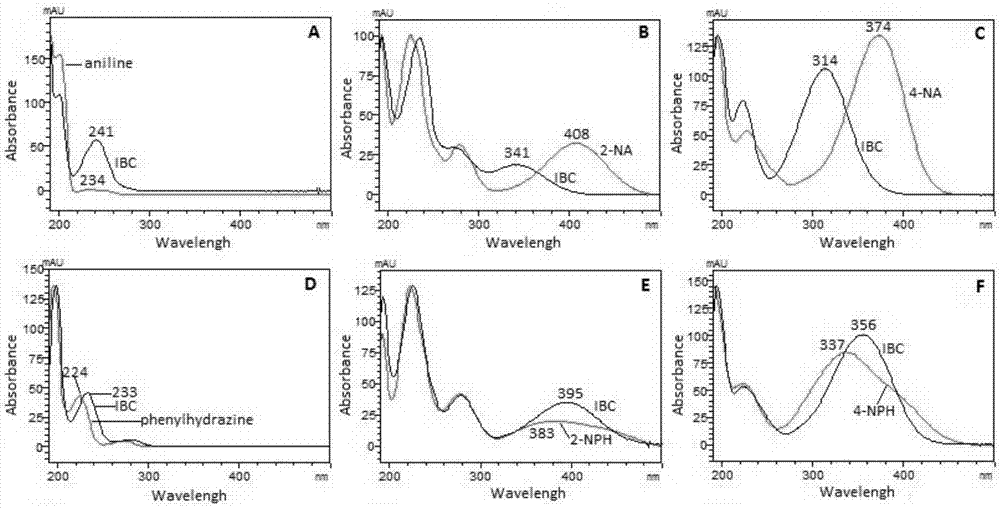
[0053] Taking isobutyryl chloride as a representative, derivatize it with the same concentration of aniline, 2-nitroaniline, 4-nitroaniline, phenylhydrazine, 2-nitrophenylhydrazine, and 4-nitrophenylhydrazine derivatization reagents. See 1.4 for derivatization experimental conditions. Under the same chromatographic conditions, use DAD to scan each derivatized product, record the spectrum and chromatogram of the product, and evaluate the pros and cons of the derivatized reagent with the maximum absorption wavelength and absorption intensity of the product.
[0054] figure 1 A shows that the maximum ultraviolet absorption wavelengths of the derivatized products of aniline and its isobutyryl chloride are 234nm and 241nm, respectively. And the maximum absorption wavelength of 2-nitroaniline and 4-nitroaniline red-shifted to 408nm ( figure 1 B) and 374nm ( figure 1 C). However, the peaks of their respective isobutyryl chloride derivatized products are blue-shifted and the absor...
Embodiment 2
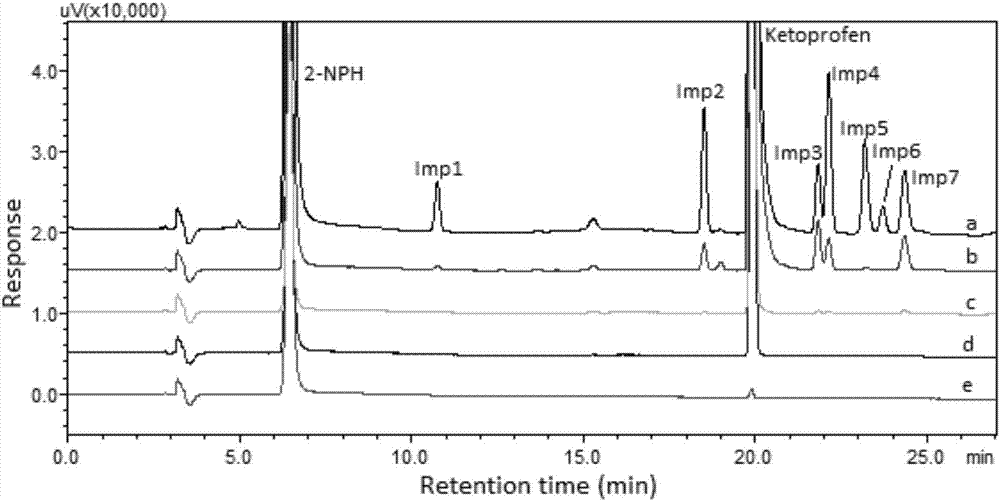
[0058] Preparation of the test solution: Accurately weigh an appropriate amount of the drug, place it in a 10mL measuring bottle, add 500 μL of derivatization reagent, add acetonitrile to dilute to the mark, shake well, and react at room temperature for 0.5h to obtain the test solution. Take 20 μL for direct injection.
[0059] Preparation of reference solution: Precisely pipette 100 μL of acid chloride stock solutions of different concentrations, put them in a 10 mL volumetric flask, add 500 μL of derivatization reagent, add acetonitrile to dilute to the mark, shake well, and react at room temperature for 0.5 h to obtain the reference solution. Take 20 μL for direct injection.
[0060] Chromatographic conditions: Shimadzu LC 20AT liquid chromatograph (including online vacuum degasser, binary gradient pump, autosampler, column thermostat, DAD detector and LC-solution chromatographic workstation); chromatographic column: Dima Technology Co., Ltd. Diamonsil TM C18 (250mm×4.6m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| linear range | aaaaa | aaaaa |
| linear range | aaaaa | aaaaa |
| linear range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com