Preparation method of absorbable haemostasis material
A technology of hemostatic materials and solid materials, which is applied in the field of preparation of absorbable hemostatic materials, can solve problems such as poor stability of cross-linking agent residues, increased difficulty of cross-linking agent removal, and unsolved problems, so as to improve tissue adhesion, Improve the anti-adhesion function and reduce the effect of tissue bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
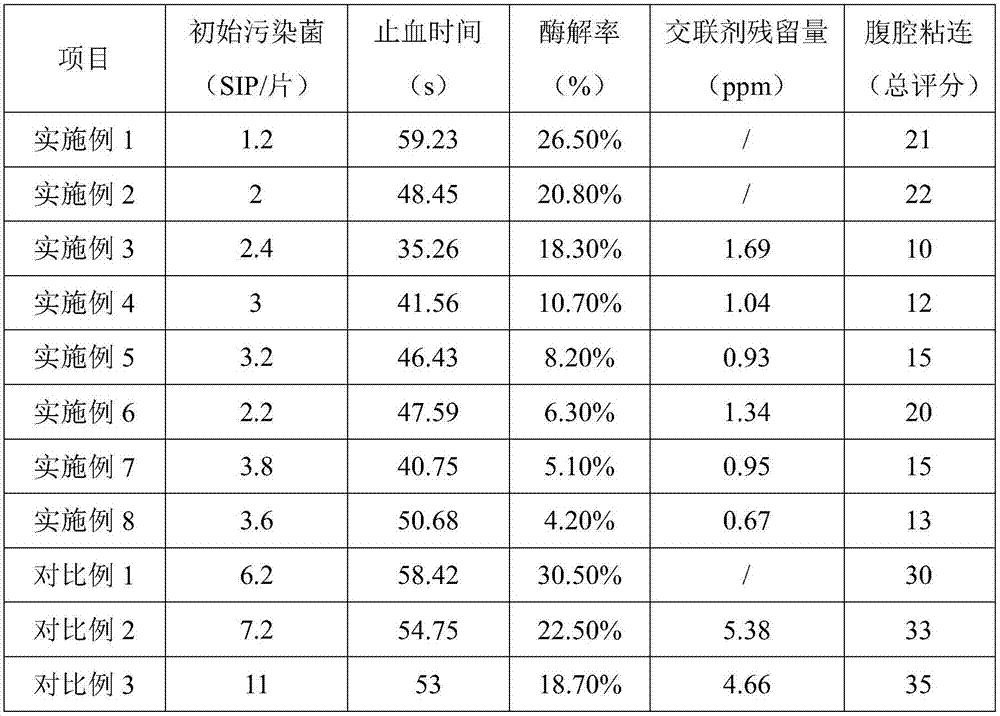
[0032] Sodium hyaluronate with a molecular weight of 3 million Daltons was dissolved in water for injection, the temperature was controlled at 4°C, the ultrasonic frequency was 20KHz, intermittently ultrasonicated for 0.5h, left to stand for 1h, and circulated twice to make hyaluronic acid with a concentration of 2.5%. Sodium bicarbonate gel solution, freeze-dried at low temperature to obtain a white material with a thickness of 2mm.
Embodiment 2
[0034] Sodium hyaluronate with a molecular weight of 1.2 million Daltons was dissolved in water for injection, the temperature was controlled at 4°C, the ultrasonic frequency was 20KHz, the ultrasonic frequency was intermittently ultrasonicated for 0.5h, left to stand for 1h, and circulated twice to make hyaluronic acid with a concentration of 1.0%. sodium bicarbonate gel solution, freeze-dried at low temperature to obtain a white material with a thickness of 5mm.
Embodiment 3
[0036] Add sodium hyaluronate with a molecular weight of 100,000 Daltons to 0.05M sodium hydroxide solution, add BDDE cross-linking agent, make the mass ratio of BDDE to sodium hyaluronate 1:40, and participate in the cross-linking reaction hyaluronic acid Sodium acid concentration is 12%, ultrasonic at 10°C, 20KHz for 0.5 hours, stand still for 2 hours, cycle reaction 3 times, use dialysis bags to dialyze the cross-linked gel, replace the dialysate regularly, remove the cross-linking agent, and then inject Wash the salt ions with water to obtain a neutral cross-linked sodium hyaluronate gel with a concentration of 2.5%.
[0037] Mix the neutral cross-linked sodium hyaluronate gel with the uncross-linked sodium hyaluronate gel (prepared in Example 1) according to the mass ratio of 9:1 to obtain a 2.5% mixed sodium hyaluronate gel, which is freeze-dried at low temperature A white material with a thickness of 3 mm was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com