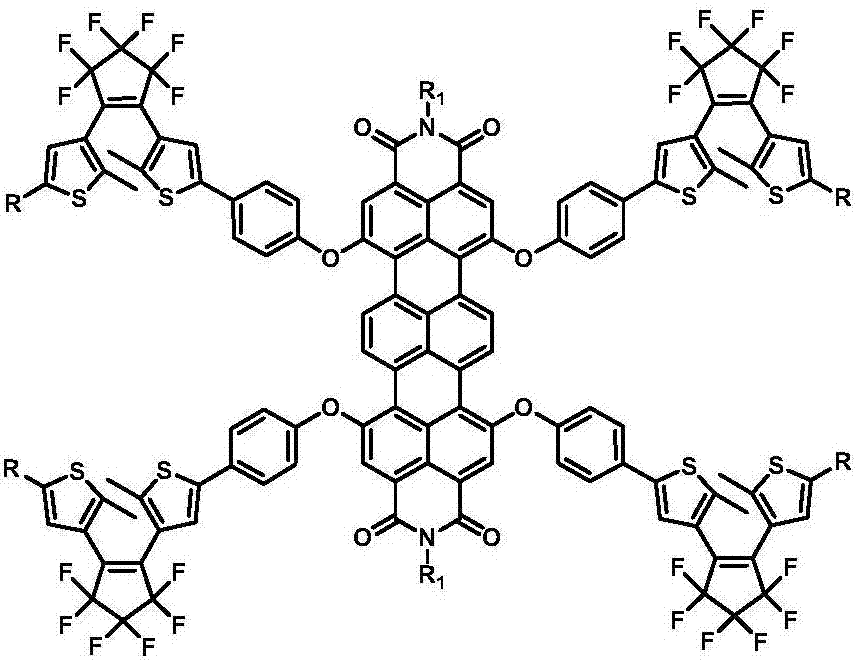
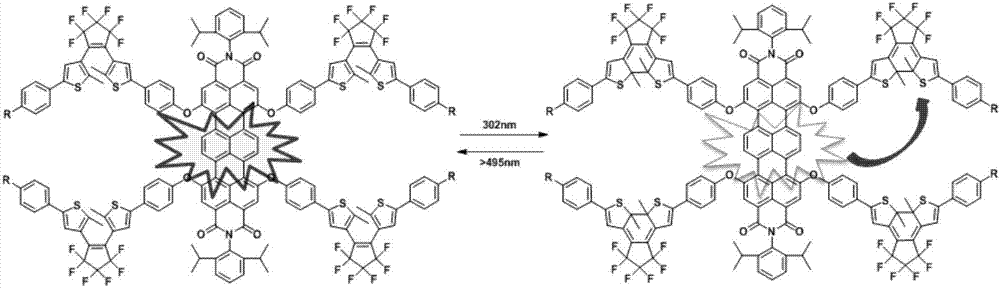
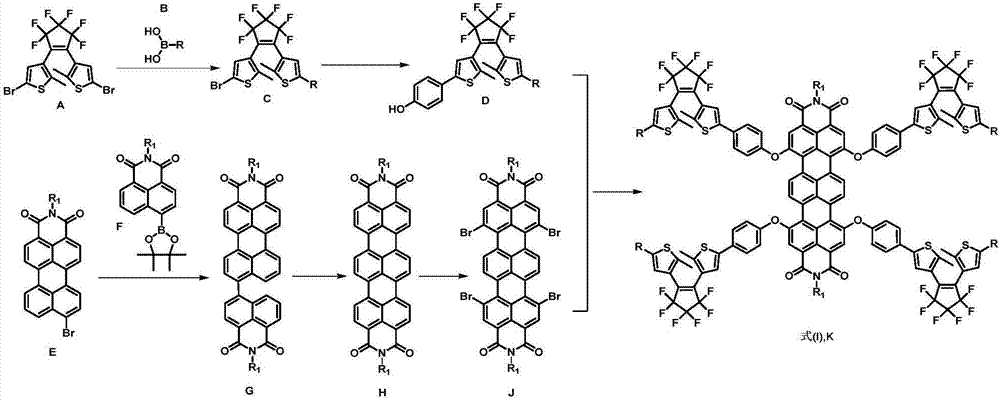
Dithienyl ethylene-trinaphthalene diphenyl imide near-infrared-fluorescence molecular switch and preparation method thereof
A technology of terrylene imide and dithienylethylene, which is applied in the field of fluorescent probes, can solve the problems of poor photostability, slow photoresponse, and low fluorescence quantum yield, and achieve good fatigue resistance and enhanced Effect of speed and quenching efficiency, high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of described four (R-dithienylethylene)-terrylene imide, comprises the steps:
[0044] (1) Compound A, compound B and sodium carbonate are uniformly dispersed in a 4:1 mixed solution of ethylene glycol dimethyl ether and water according to a molar ratio of 1:1:5, and under nitrogen protection, a catalyst dose of Pd (PPh 3 ) 4 , heated to 80-100 DEG C for 12-24 hours, after the completion of the reaction, separation and purification to obtain compound C, the molecular structures of A, B and C are shown in formula (A), formula (B) and formula (C);
[0045]
[0046] (2) The compound C, p-hydroxyphenylboronic acid and sodium carbonate are uniformly dispersed in a 4:1 mixed solution of ethylene glycol dimethyl ether and water according to a molar ratio of 1:1:5, and under nitrogen protection, add Catalyst dosage of Pd(PPh 3 ) 4 , heated to 80-100°C and reacted for 12-48 hours. After the reaction was completed, the compound D was obtained after s...
Embodiment 1
[0060] A near-infrared fluorescent molecular switch shown in formula (I), wherein the substituent R is R 1 for
[0061]
[0062] Its synthetic route is as follows image 3 Shown, preparation method comprises the steps:
[0063] (1) 1-(5-bromo-2-methylthiophen-3-yl)-2-[2-methyl-5-(4-octyloxyphenyl)thiophen-3-yl]perfluorocyclopentyl Synthesis of alkenes (compound C, structural formula shown in formula (C)).
[0064] Add 1,2-bis(5-bromo-2-methylthiophen-3-yl)perfluorocyclopentene (1.58g, 3mmol), 4-octyloxyphenylboronic acid (0.75 g, 3mmol), anhydrous sodium carbonate (1.59g, 15mmol), water (6ml) and ethylene glycol dimethyl ether (DME, 24ml) and stirred with a magnetic force, blowing nitrogen into the mixture for 30min to fully remove the solvent and the reaction system of oxygen. Then add the zero-valent palladium catalyst Pd(PPh 3 ) 4 (0.17g, 0.15mmol), immediately use a double-row tube to carefully evacuate nitrogen three times, so that the entire system is stric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com