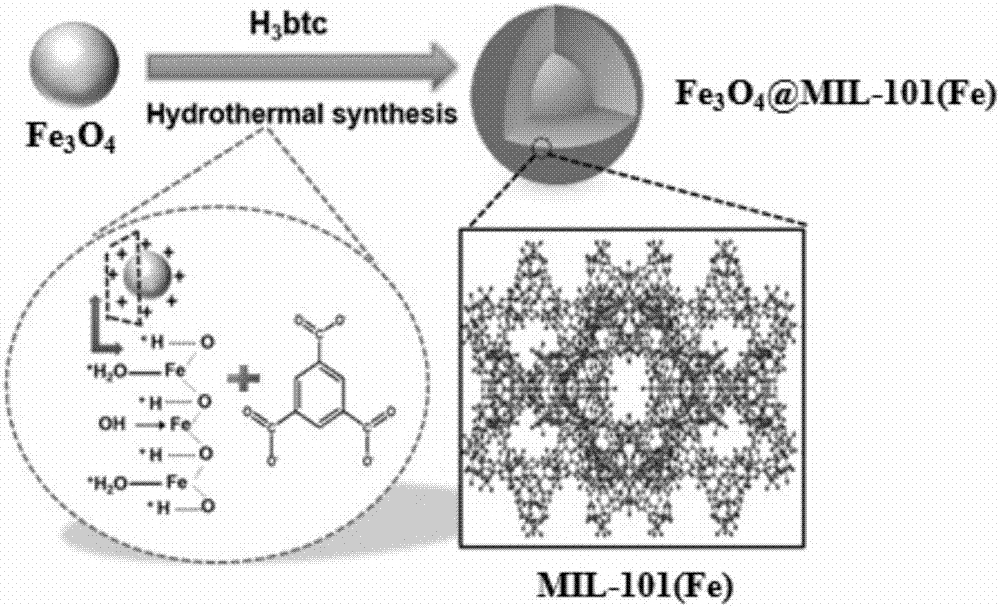
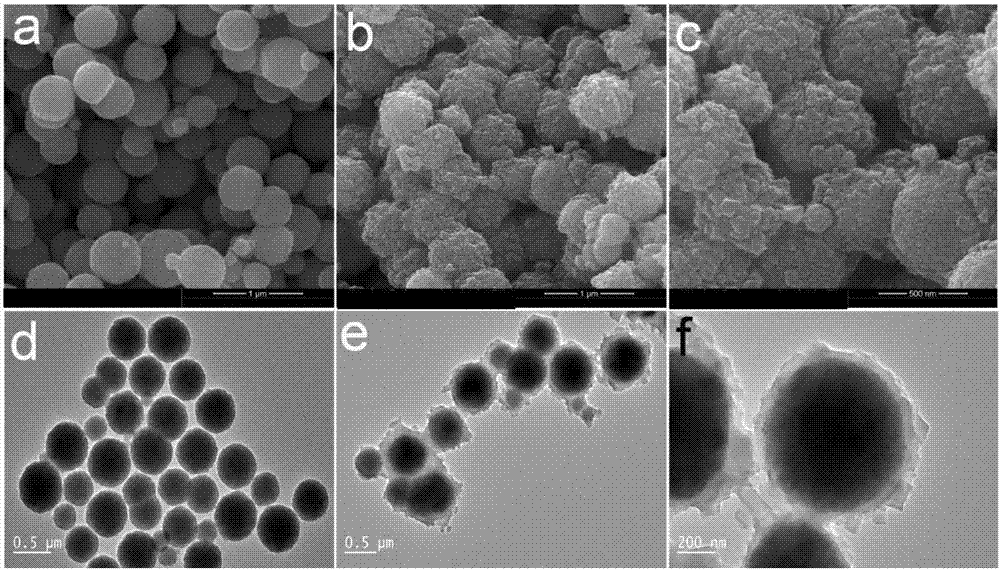
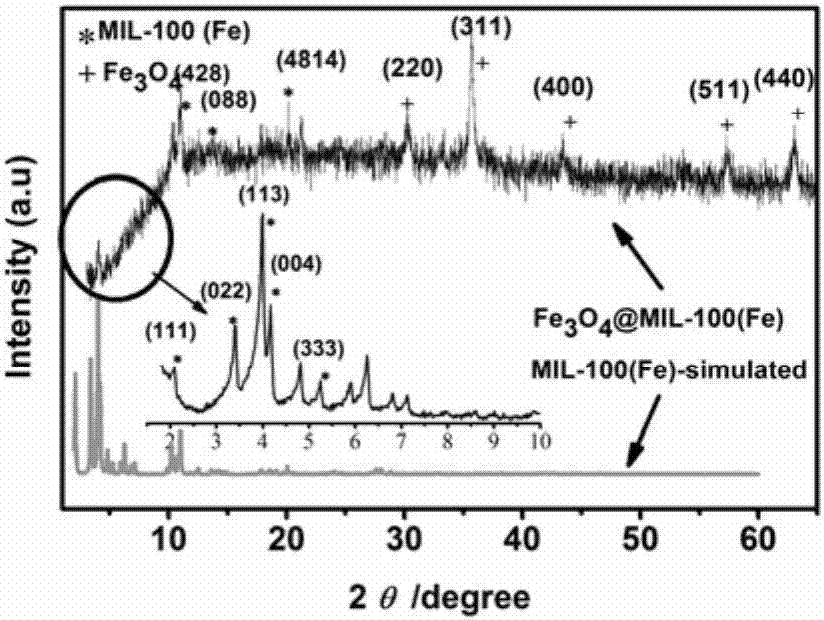
Method for in-situ hydrothermal preparation of magnetic metal-organic framework core-shell material
A technology of magnetic metals and organic frameworks, applied in the fields of alkali metal compounds, chemical instruments and methods, alkali metal oxides/hydroxides, etc., can solve the problems of cumbersome layer-by-layer self-assembly, and achieve simple synthesis methods and easy recycling Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the Fe prepared by the present invention is given below 3 o 4 Adsorption efficiency diagram of 7 organic dyes on @MIL-101(Fe) material. From Figure 8 It can be seen that the material has adsorption effect on 7 kinds of dyes, and the order of adsorption efficiency is: methylene blue>basic blue 6B>rhodamine B>acid chrome blue K>chrome black T>indigo>acid magenta, and the adsorption amount is between Between 42.0-221mg g -1 Between, this result shows that Fe 3 o 4 The @MIL-101(Fe) material can be used for the removal of dyes in water and can be used to improve water quality.
Embodiment 2
[0032] Embodiment 2: the Fe prepared by the present invention is given below 3 o 4 Adsorption behavior of methylene blue on @MIL-101(Fe) material. The adsorption behavior was studied by Langmuir and Freundlich two isotherm adsorption models. Fe at different temperatures 3 o 4 See Table 1 for the isothermal adsorption parameters of @MIL-101(Fe) to methylene blue. Table 1 shows that the Freundlich isothermal adsorption model is used to linearly fit its adsorption behavior, and the linear regression coefficient is only 0.895, while the Langmuir model fitted The linear regression coefficient reached 0.9976, indicating that its adsorption behavior conformed to the Langmuir isotherm adsorption model.
[0033] Table 1
[0034]
Embodiment 3
[0035] Embodiment 3: the Fe prepared by the present invention is given below 3 o 4 The change value of the adsorption efficiency of @MIL-101(Fe) material after several cycles. Figure 9 showed that after 4 cycles, Fe 3 o 4 The methylene blue adsorption efficiency of @MIL-101(Fe) material is still 154.7mg g -1 , keeping 70% of the original efficiency, which shows that it has good recycling efficiency.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com