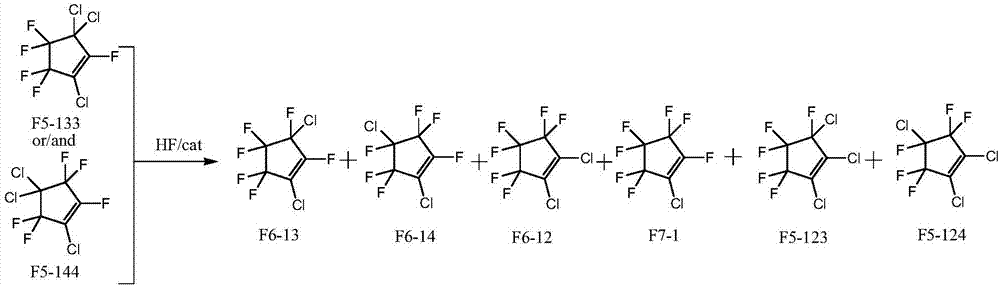
Method for simultaneously preparing dichlorohexafluorocyclopentene isomers
A technology for dichlorohexafluorocyclopentene and trichloropentafluorocyclopentene is applied in the field of simultaneous preparation of dichlorohexafluorocyclopentene isomers, and can solve problems such as being difficult to exist stably, unattractively, and difficult to find, etc. problem, to achieve the effect of stable activity and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of fluorination catalyst: dissolve chromium nitrate in water, add precipitant ammonia water at 60°C, control the pH of the solution to be in the range of 7.5 to 8.5, make it fully precipitate under stirring conditions, filter the formed slurry, use Ionized water was washed to neutrality, and then dried at 150° C. for 12 hours to obtain chromium hydroxide. The above-mentioned chromium hydroxide and cobalt hydroxide are uniformly mixed according to the mass percentage of 95% and 5%, and pressed into shape to obtain a catalyst precursor. Activated at 300° C. with a mixed gas composed of hydrogen fluoride and hydrogen with a molar ratio of 10:1 for 10 hours to prepare a fluorination catalyst.
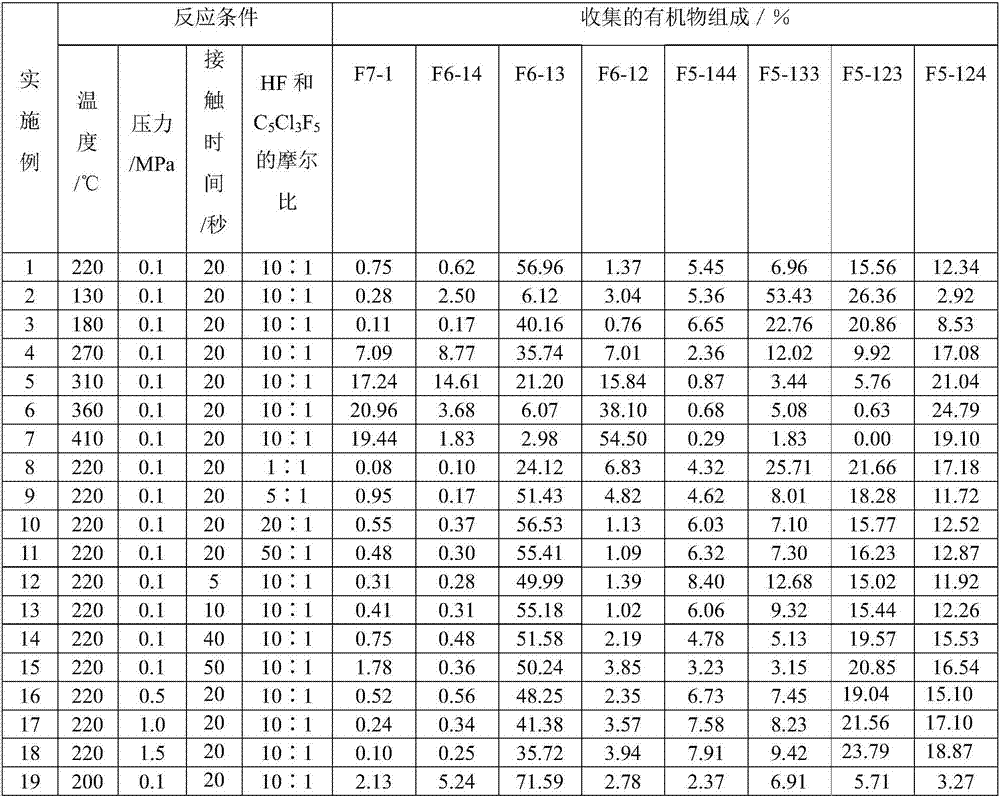
[0031] 10 ml of the fluorination catalyst prepared above was charged in a tubular reactor made of Inconne with an inner diameter of 1 / 2 inch and a length of 30 cm. The temperature of the reactor was raised to 220°C, under the conditions that the molar ratio of hydrogen flu...
Embodiment 2
[0033] The same operation as in Example 1, the difference is that the reaction temperature is changed to 130° C. The results are shown in Table 1.
Embodiment 3
[0035] The same operation as Example 1, the difference is that the reaction temperature is changed to 180 ° C, the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com