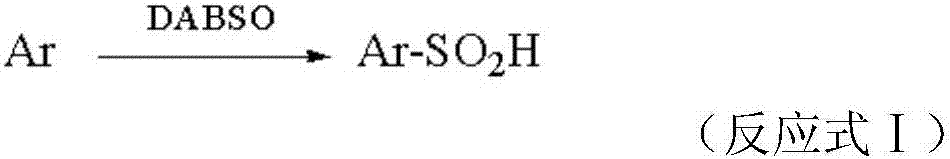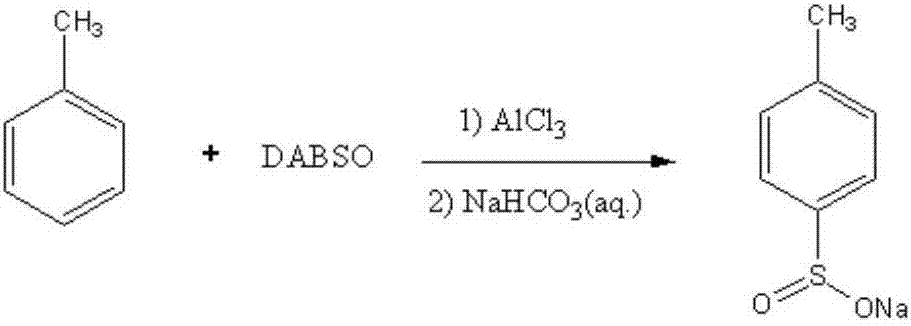Preparation method of aromatic sulfinic acid compound
A technology for aromatic sulfinic acid and aromatic compounds, applied in a new field of preparation, can solve the problems of harsh reaction conditions and expensive catalysts, achieve mild reaction conditions, omit chlorosulfonation reaction steps, and facilitate storage and transportation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1:4-sodium methylbenzene sulfinate
[0024] 1) DABSO (1,4-diazabicyclo[2.2.2]octane and SO 2 The preparation of the adduct):
[0025] In a 100mL three-necked flask connected with a tail gas absorption device, add 50mmol of 1,4-diazabicyclo[2.2.2]octane and 50mL of dichloromethane in sequence, and then slowly introduce 60mmol of SO at room temperature. 2 Gas, react at room temperature for 4 hours after passing through, filter with suction, dry, and store the obtained solid for later use;
[0026] 2) Preparation of sodium 4-methylbenzenesulfinate:
[0027] Under normal pressure, into a 100mL three-necked flask with mechanical stirring, add 40mL of dichloromethane, 8.0mmol of toluene, 25.0mmol of aluminum trichloride, and 8.2mmol of the above-mentioned self-made DABSO at 20°C, and then heat up to 25-30°C, react for 8-10 hours, and when the liquid chromatography detects that the toluene content in the reaction solution is lower than 10% and ...
Embodiment 2
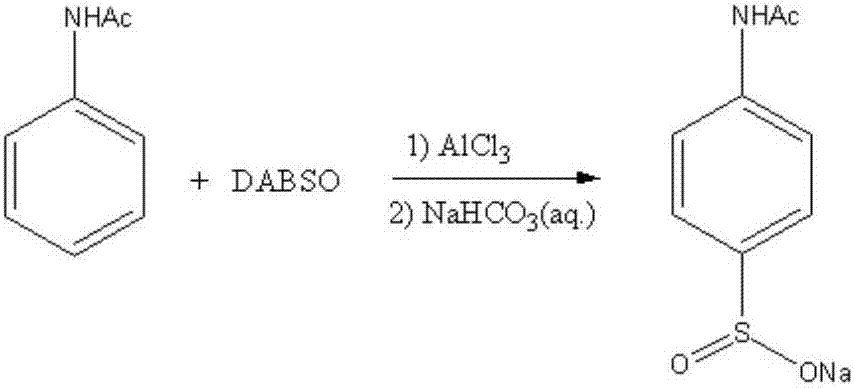
[0031] Embodiment 2: the preparation of sodium 4-acetamidobenzenesulfinate
[0032] DABSO (1,4-diazabicyclo[2.2.2]octane and SO 2 The preparation of the adduct) is the same as in Example 1, the same below.
[0033] Under normal pressure, add 20mL of 1,2-dichloroethane, 3.0mmol of N-acetanilide, 24.0mmol of aluminum trichloride, 3.0mmol of the above self-made DABSO, then heated up to 25-40°C, reacted for 20-24h, and when the N-acetanilide content in the reaction liquid was detected by liquid chromatography to be lower than 1%, the reaction ended. The post-processing operation was the same as in Example 1 to obtain 0.53 g of white solid powder of sodium 4-acetamidobenzenesulfinate with a yield of 80%. Its reaction formula is as follows:
[0034]
[0035] The NMR data are as follows:
[0036] Sodium 4-acetamidobenzenesulfinate: 1 H NMR (400MHz, Methanol-d 4 ,δ):7.67-7.63(d,2H,aromatic),7.62-7.58(d,2H,aromatic),2.15(s,3H,COCH 3 ).
Embodiment 3
[0037] Embodiment 3: the preparation of sodium 4-chlorobenzenesulfinate
[0038]Under normal pressure, add 25mL of dichloromethane, 6.2mmol of chlorobenzene, 18.8mmol of aluminum trichloride, and 6.2mmol of the above-mentioned self-made DABSO at 25°C to a 100mL three-necked flask with mechanical stirring, and then heat up to 30-35°C, react for 12-14 hours, and when the liquid chromatographic detection shows that the chlorobenzene content in the reaction solution is lower than 5% and no longer decreases, the reaction ends. The post-processing operation was the same as that of Example 1, and 1.08 g of white solid powder of sodium 4-chlorobenzenesulfinate was obtained, with a yield of 88%. Its reaction formula is as follows:
[0039]
[0040] The NMR data are as follows:
[0041] Sodium 4-chlorobenzenesulfinate: 1 H NMR (400MHz, Methanol-d 4 ,δ):7.68-7.60(m,2H,aromatic),7.46-7.42(m,2H,aromatic).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com