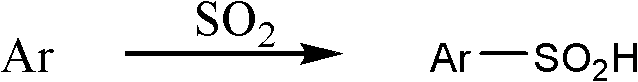Aromatic sulfinic acid compound preparation method
A technology for aromatic sulfinic acid and aromatic compounds, which is applied in the field of preparation of aromatic sulfinic acid compounds, can solve the problems of low reaction temperature, large consumption, harsh reaction conditions and the like, achieves mild reaction conditions and simplifies experimental operation. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 α-naphthalenesulfinic acid
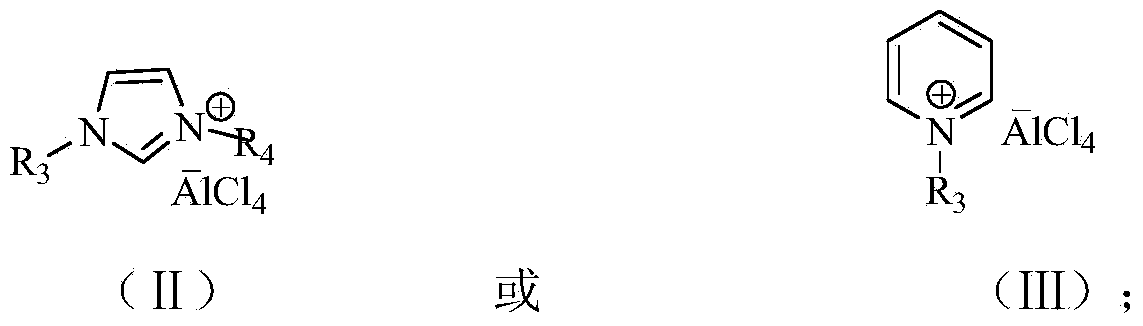
[0034] 1) Ionic liquid 1-butyl-3-methylimidazolium chloroaluminate [Bmim][AlCl 4 ] Catalyst preparation:
[0035]Synthesis of ionic liquid precursor 1-n-butyl-3-methylimidazolium chloride [Bmim]Cl: take N-methylimidazole dried with phosphorus pentoxide and 1-chlorobutane at a molar ratio of 1:2 , and react at a constant temperature at 70-72°C for 48 hours, follow the complete reaction of N-methylimidazole by liquid chromatography, distill off excess 1-chlorobutane under reduced pressure, cool and place, and store the obtained solid for later use.
[0036] Ionic liquid [Bmim] [AlCl 4 ] Synthesis: Weigh [Bmim]Cl and AlCl respectively 3 Solid, the molar ratio of the two is 1:2, slowly add AlCl in batches to [Bmim]Cl at 50°C 3 , stirred at 100°C for 12h, the prepared [Bmim][AlCl 4 ] The ionic liquid was stored in a desiccator.
[0037] 2) Preparation of α-naphthalenesulfinic acid:
[0038] Under normal pres...
Embodiment 2
[0040] The preparation of embodiment 2 benzene sulfinic acid
[0041] 1) Ionic liquid 1-(1-tetradecyl)-3-methylimidazolium chloroaluminate [Tmim][AlCl 4 ] Catalyst preparation:
[0042] Synthesis of ionic liquid precursor 1-(1-tetradecyl)-3-methylimidazolium chloride [Tmim]Cl: N-methylimidazole and tetradecane chloride in a molar ratio of 1:1.6, in The reaction was carried out at a constant temperature of 95°C for 72 hours. Liquid chromatography showed no N-methylimidazole, so the reaction was stopped, tetradecane chloride was evaporated under reduced pressure, and cooled to room temperature for use.
[0043] Ionic liquid [Tmim] [AlCl 4 ] Synthesis: Weigh [Tmim]Cl and AlCl respectively 3 Solid, the molar ratio of the two is 1:2, slowly add AlCl in batches to [Tmim]Cl at 60°C 3 , stirred at 100°C for 12h, the prepared [Tmim][AlCl 4 ] The ionic liquid was stored in a desiccator.
[0044] 2) Preparation of benzenesulfinic acid:
[0045] In a 100ml four-necked bottle connec...
Embodiment 3
[0047] The preparation of embodiment 3 4-methylbenzenesulfinic acid
[0048] Ionic Liquid 1-Butyl-3-Methylimidazolium Chloroaluminate [Bmim][AlCl 4 ] The preparation of catalyst is with embodiment 1.
[0049] In a 100ml four-necked bottle connected with a tail gas receiving device, add 0.5mol toluene and 1.1mol of the above-mentioned self-made ionic liquid [Bmim][AlCl 4 ], down to -20°C, slowly add SO 2 The liquid is 0.6 mol, raised to 20-25°C, and reacted for 2-3 hours. At this time, liquid chromatography detection shows that the content of raw materials no longer decreases (about 7% toluene is unreacted), stop stirring, and the reaction ends. The organic phase was extracted with ethyl acetate, and the remaining ionic liquid was reused. The post-processing operation was the same as that in Example 1 to obtain 69.6 g of 4-methylbenzenesulfinic acid yellow powder with a purity of 92% and a yield of 82%. The NMR data are as follows:
[0050] 4-Methylbenzenesulfinic acid: 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com