Drug-eluting stent
A technology for eluting stents and drugs, used in stents, drug combinations, drug delivery, etc., can solve problems such as complex processes, and achieve the effects of improving restenosis, inhibiting intimal thickening, and high coating strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] A cobalt-chromium alloy as the base member 2 is coated with a mixture of cilostazol and one of the polymers listed above (e), (j) and (p) by ultrasonic spraying, wherein cilostazol and The mixing ratio of the polymers was varied as shown in Table 2, and the strength of each coating was evaluated. The results are shown in Table 2. In the table, the symbol "○" indicates that the intensity is very high, the symbol "Δ" indicates that the intensity is high, and the symbol "×" indicates that the intensity is low.
[0099] The results show that when the mixing ratio (D / P ratio) of cilostazol and polymer is 6:4 or lower, that is, when the amount of cilostazol is smaller than this ratio, the strength is high, and particularly, when the With a ratio of 5:5, the intensity is high enough. However, when the D / P ratio is less than 4:6, it is presumed that the drug effect of cilostazol for drug-eluting stents may be reduced. Therefore, the D / P ratio is preferably 4:6 or higher.
...
Embodiment 2
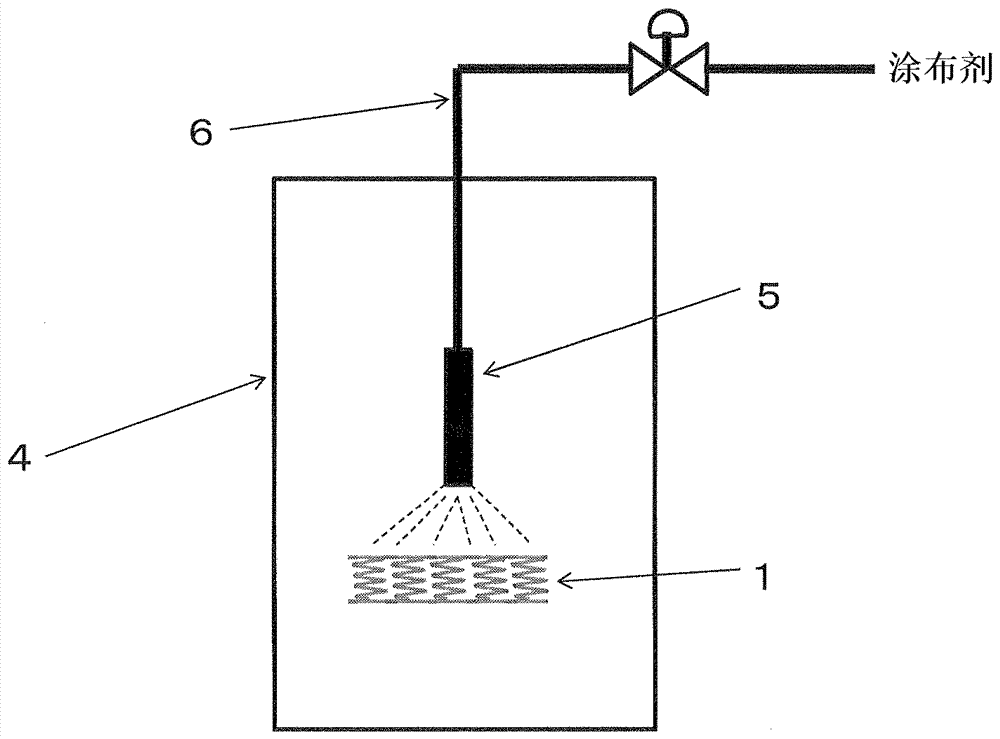
[0103] A cobalt-chromium alloy as the base member 2 was coated with a coating agent prepared by mixing cilostazol and one of the 17 kinds of polymers (a) to (q) listed in Table 1 by ultrasonic spraying. The mixing ratio of cilostazol and each polymer is 5:5.
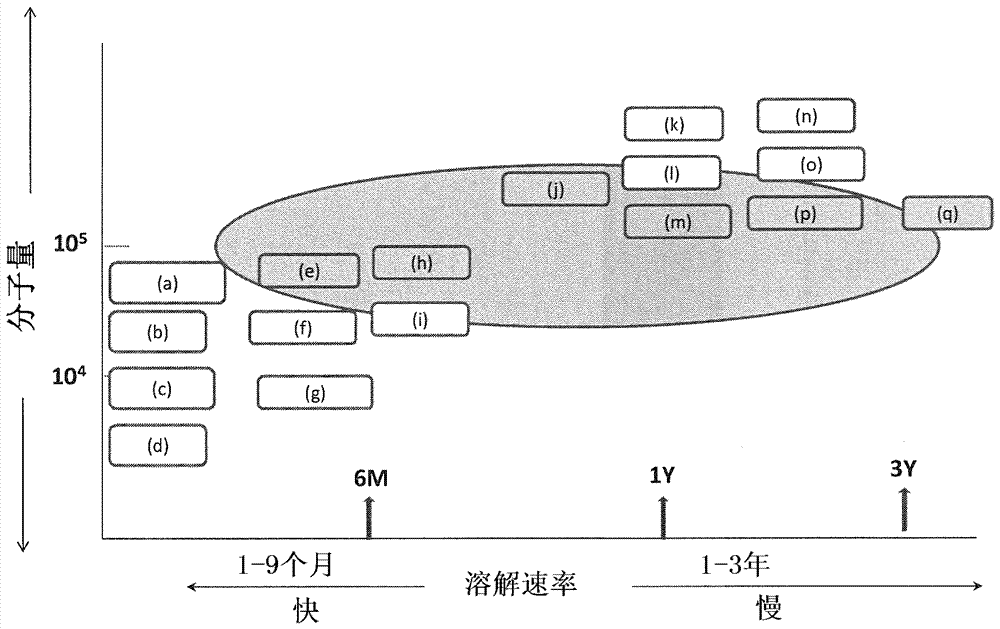
[0104] The appearance of each prepared stent was observed in terms of coating work. On external observation, in the coated stent there is no evidence of image 3 The meshes in the close together or without are shown in the Figure 4 A coated stent with uneven coating and a surface that was flat and smooth, not like orange peel, was rated as "good". The results are shown in Figure 5 middle. exist Figure 5 In , the axis of coordinates represents the molecular weight of each polymer mixed with cilostazol, and the axis of abscissa represents the dissolution rate of the coating agent. displayed on Figure 5 (a) to (q), which are the 17 polymers listed in Table 1, show the relationship of the two factors by their posi...
Embodiment 3
[0107] Scaffolds were prepared in a similar manner to Example 2 using the above polymers (e), (h), (j), (m), (p) and (q). As explained below, each prepared stent was placed in the iliac vessel of a rabbit, and the effect of each stent on inhibiting intimal thickening was evaluated.
[0108] First, the rabbit's neck was cut open, and the right carotid artery was taken out, and an introducer was connected thereto. The guide wire of the balloon catheter is inserted from the introducer and moved under X-ray fluoroscopy to the distal part of the iliac artery to be treated. And then, an angiographic catheter is inserted along the guide wire, and angiography is performed at the treated site of the iliac artery. After performing angiography at the treatment site, a balloon catheter with a test stent was inserted into the treatment site under X-ray fluoroscopy along the guide wire of the balloon catheter. The test stent (where the stent diameter is 2.75 mm when the standard diameter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com