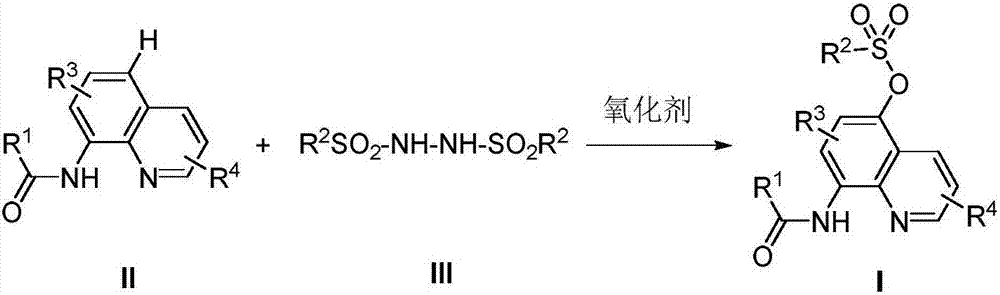
Preparation method of 5-sulfonyl oxy-8-carboxamidoquinoline derivative
A technology of sulfonyloxy and quinoline, which is applied in the preparation of 5-sulfonyloxy-8-amidoquinoline derivatives, and in the field of preparing 5-sulfonyloxy-8-acetamidoquinoline, can Solve the problems of high price, low yield, and many reaction steps, and achieve the effect of wide substrate adaptation, easy availability of raw materials, and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
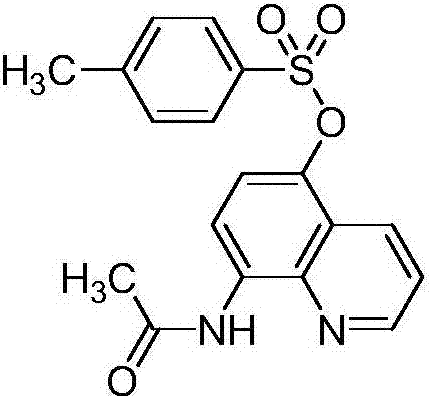
[0015] Preparation of 5-(4-methylbenzenesulfonyloxy)-8-acetamidoquinoline (I-1)
[0016]
[0017] Add tetrahydrofuran (100mL), 8-acetamidoquinoline (II-1) (1.86g, 0.01mol), N,N'-bis(4-methylbenzenesulfonyl)hydrazine (III- 1) (3.70g, 0.011mol) and iodobenzene trifluoroacetate (14.2g, 0.033mol), stirred at room temperature for 1 hour, filtered, washed the filter cake with dichloromethane, and removed the solvent under reduced pressure to obtain the crude product. Column chromatography ( Petroleum ether / ethyl acetate as eluent, gradient elution) to obtain white solid 2.78g, yield 78%, mp163-167°C. 1 H NMR(300MHz, CDCl 3 )δ(ppm): 9.78(s,1H), 8.85(d,J=2.8Hz,1H), 8.65(d,J=8.6Hz,1H), 8.44(d,J=8.4Hz,1H), 7.79 (d, J = 8.1 Hz, 2H), 7.53 (dd, J = 8.4, 4.2 Hz, 1H), 7.36 (d, J = 7.9 Hz, 2H), 7.01 (d, J = 8.6 Hz, 1H), 2.49 (s,3H),2.38(s,3H); 13 C NMR(75MHz, CDCl 3 )δ(ppm): 168.27, 148.29, 145.29, 138.95, 133.31, 131.67, 130.84, 129.44, 128.14, 126.77, 122.41, 121.60, 119.31, 114.62, 24.52, 21...
Embodiment 2
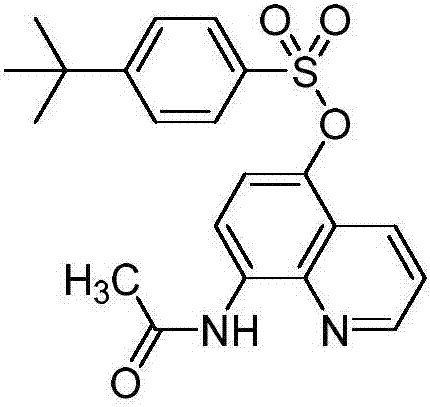
[0019] Preparation of 5-(4-tert-butylbenzenesulfonyloxy)-8-acetamidoquinoline (I-2)
[0020]
[0021] Add tetrahydrofuran (100mL), II-1 (1.86, 0.01mol), N,N'-bis(4-tert-butylbenzenesulfonyl)hydrazine (III-2) (4.66g, 0.011mmol) into a 250mL four-necked flask And iodobenzene trifluoroacetate (14.2g, 0.033mmol), stirred at room temperature for 1 hour, filtered, washed the filter cake with dichloromethane, and removed the solvent under reduced pressure to obtain the crude product. Column chromatography (petroleum ether / ethyl acetate as elution Reagent, gradient elution) to obtain 2.71 g of white solid, yield 68%, mp142-145°C. 1 H NMR(300MHz, CDCl 3 )δ(ppm): 9.74(s,1H),8.81(s,1H),8.66(d,J=8.4Hz,1H),8.33(d,J=8.5Hz,1H),7.81(d,J= 7.1Hz, 2H), 7.55(d, J=7.1Hz, 2H), 7.47(s, 1H), 7.08(d, J=7.5Hz, 1H), 2.37(s, 3H), 1.36(s, 9H) ; 13 C NMR(75MHz, CDCl 3 )δ (ppm): 168.26, 158.30, 148.20, 138.91, 137.83, 133.30, 131.59, 130.74, 127.95, 125.82, 122.38, 121.49, 119.46, 114.70, 34.87, 30.48, 24.52....
Embodiment 3
[0023] Preparation of 5-benzenesulfonyloxy-8-acetamidoquinoline (I-3)
[0024]
[0025] Using II-1 (1.86g, 0.01mol), N,N'-diphenylsulfonylhydrazine (III-3) (3.43g, 0.011mmol) and iodobenzene trifluoroacetate (14.2g, 0.033mmol) as raw materials. The operation is the same as that in Example 1, to obtain 2.46 g of white solid, with a yield of 72%, m.p. 165-168°C. 1 H NMR(300MHz, CDCl 3 )δ(ppm): 9.77(s,1H), 8.85(d,J=4.3Hz,1H), 8.67(d,J=8.6Hz,1H), 8.39(d,J=8.4Hz,1H),7.92 (d,J=7.3Hz,2H), 7.72(t,J=7.4Hz,1H), 7.60–7.48(m,3H), 7.04(d,J=8.6Hz,1H), 2.38(s,3H) ; 13 C NMR(75MHz, CDCl 3 )δ(ppm): 168.33,148.33,138.34,137.61,134.49,134.07,130.67,128.86,128.10,122.29,121.67,119.40,119.30,114.59,24.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com