A preparation method of a high -purity injection for cephalosporin sodium
A technology for cefotetan disodium and injection, which is applied in the production and purification process of high-purity injection drug compounds, and the field of preparation of cefotetan disodium, which can solve the problems of affecting product quality and easy breakage, so as to avoid The effect of quality risk, reaction thoroughness, reaction efficiency and conversion rate improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] A kind of preparation method of high-purity cefotetan disodium for injection of the present invention, its specific implementation steps are as follows:
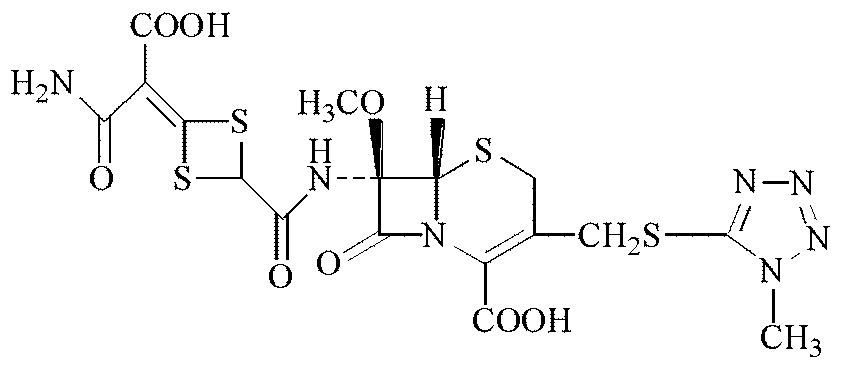
[0035]a. Under the protection of nitrogen, the reaction raw material 7-MAC (chemical name 7β-amino-7α-methoxy-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephalosporin Diphenylmethyl ene-4-carboxylate) was dissolved in dichloromethane as a solvent, cooled to -25~-40°C, added pyridine as a catalyst, and then added dichloromethane solution of acylating agent, stirred evenly and acylated Reaction, the reaction temperature is controlled at -20 to -40°C, and the reaction time is 15 to 45 minutes; the acylating agent is any one or a mixture of chloroacetyl chloride or bromoacetyl bromide.
[0036] b. Adjust the pH of the solution after the reaction in step a to below 1.0 with dilute sulfuric acid or dilute hydrochloric acid. After the solution is allowed to stand for phase separation, separate the dichloromethane phase, add pu...
Embodiment 1
[0042] a. Under the protection of nitrogen, dissolve the reaction material 7-MAC in the solvent methylene chloride, cool down to -38°C, add pyridine as a catalyst, and then add the methylene chloride solution of acylating agent chloroacetyl chloride, stir evenly and carry out Acylation reaction, the reaction temperature is controlled at -35°C, and the reaction time is 30 minutes;
[0043] b. Adjust the pH of the solution after the reaction in step a to 0.5 with dilute sulfuric acid or dilute hydrochloric acid. After the solution is allowed to stand for phase separation, the dichloromethane phase is separated, and purified water is added to the dichloromethane phase, and the dichloromethane phase is separated again. phase and separate the dichloromethane phase, add anhydrous magnesium sulfate to the dichloromethane phase that has been separated again, stir well and filter, remove the filter residue, and add n-butanol, dichloromethane, Ethyl acetate and tetrahydrofuran were used...
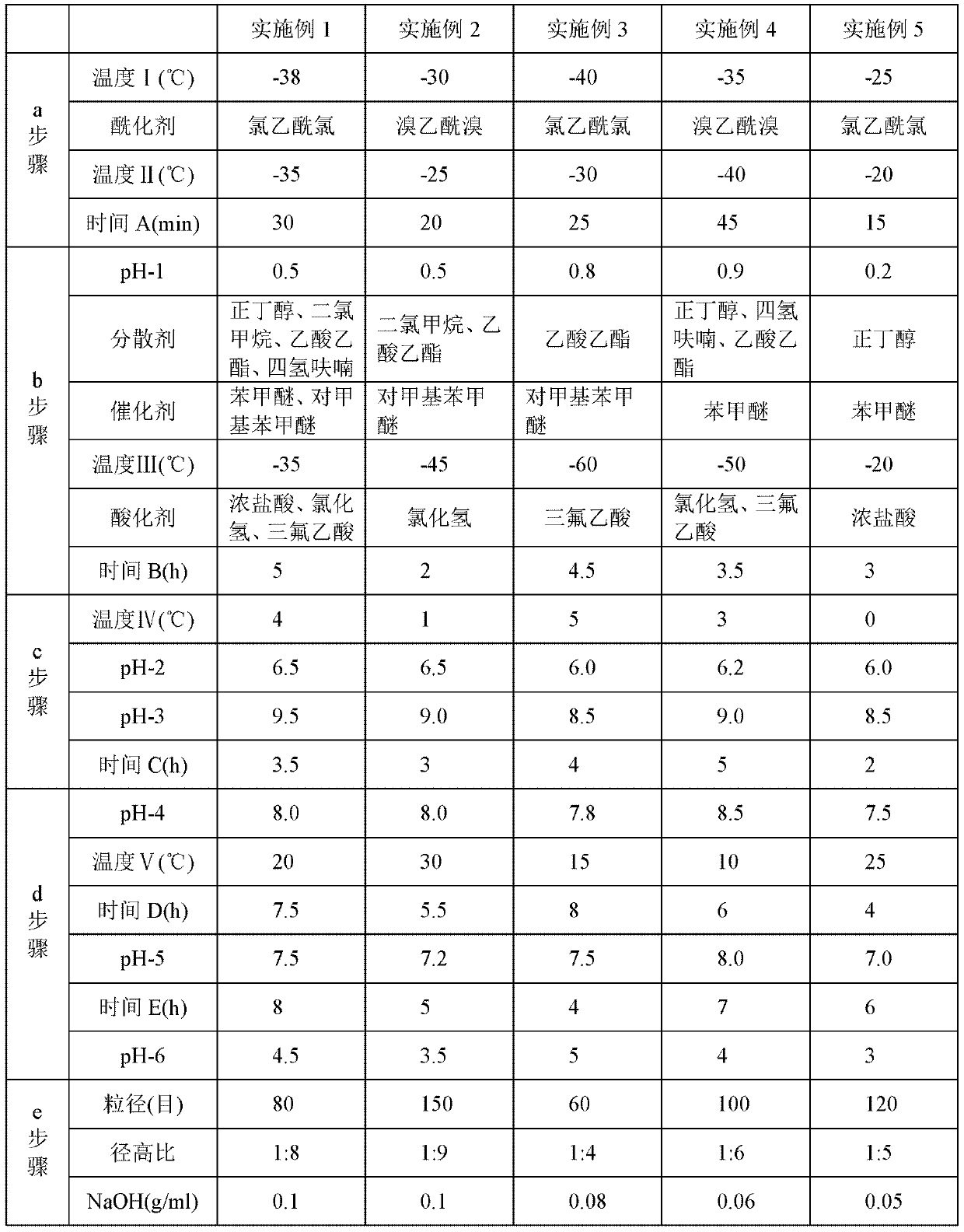
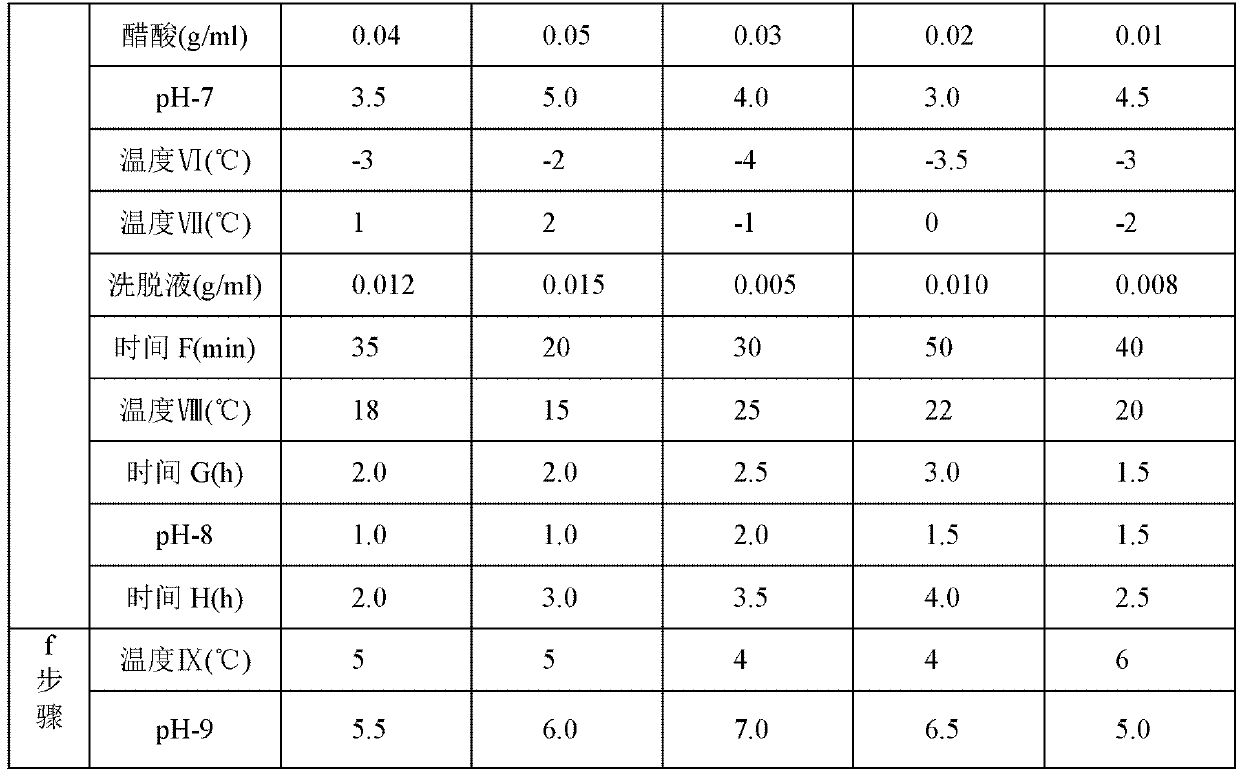
Embodiment 2~5
[0049] The steps of the preparation method adopted in Examples 2 to 5 are the same as those in the above-mentioned Example 1, but the different process parameters or selected different types of compounds are shown in Table 1 below. Wherein, in step a, "temperature I" represents the temperature reached by cooling after 7-MAC is dissolved in solvent methylene chloride; "temperature II" represents the temperature during the acylation reaction; "time A" represents the time of the acylation reaction; step b Among them, "pH-1" represents the pH value of the solution after the reaction in step a is adjusted with dilute sulfuric acid or dilute hydrochloric acid; "temperature III" represents the temperature reached after adding the catalyst; "time B" represents the time of the hydrolysis reaction; In step c, "temperature IV" represents the temperature after adding ice water; "pH-2" represents the pH value of the solution before static phase separation; "pH-3" represents the pH value of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com