Method for electrochemically detecting activation level of STAT 3 protein through DNA probe ligand and application
A DNA probe and electrochemical technology, applied in the fields of molecular diagnosis and obstetric high-risk pregnancy detection, can solve problems such as affecting new signal amplification strategies, poor universality of detection systems, and difficulty in meeting life sciences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
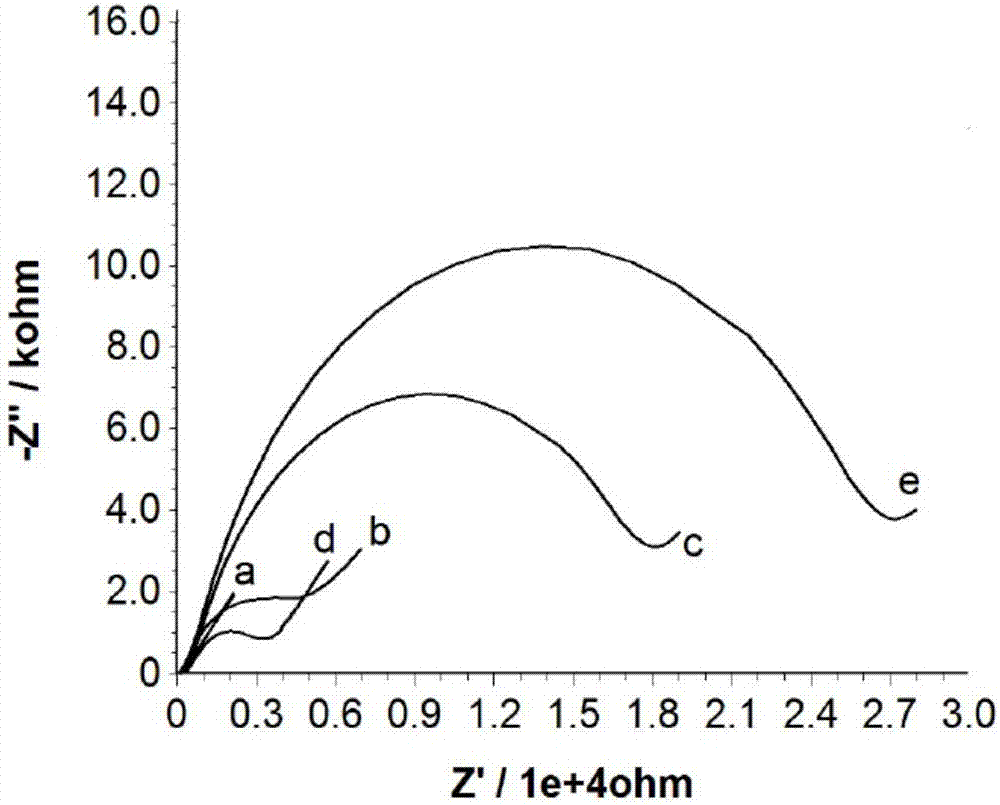
[0059] AC impedance is an effective means to study the interface characteristics of surface-modified electrodes. In order to confirm the modification of the electrode surface, the change of the surface state of the gold electrode was first studied by EIS technique at various stages of detection. The AC impedance plot includes a semicircle in the high-frequency region associated with the interfacial electron transfer process, and a linear portion in the low-frequency region associated with the bulk solution diffusion process to the electrode surface. The increase of the diameter of the semicircle reflects the increase of the charge transfer resistance on the electrode surface.
[0060] figure 1 It clearly reflects the AC impedance on the surface of the gold electrode in different reaction stages. like figure 1 As shown in a, the impedance diagram of the bare gold electrode is almost a straight line, indicating that the transfer of electrons on the electrode surface has not b...
Embodiment 2
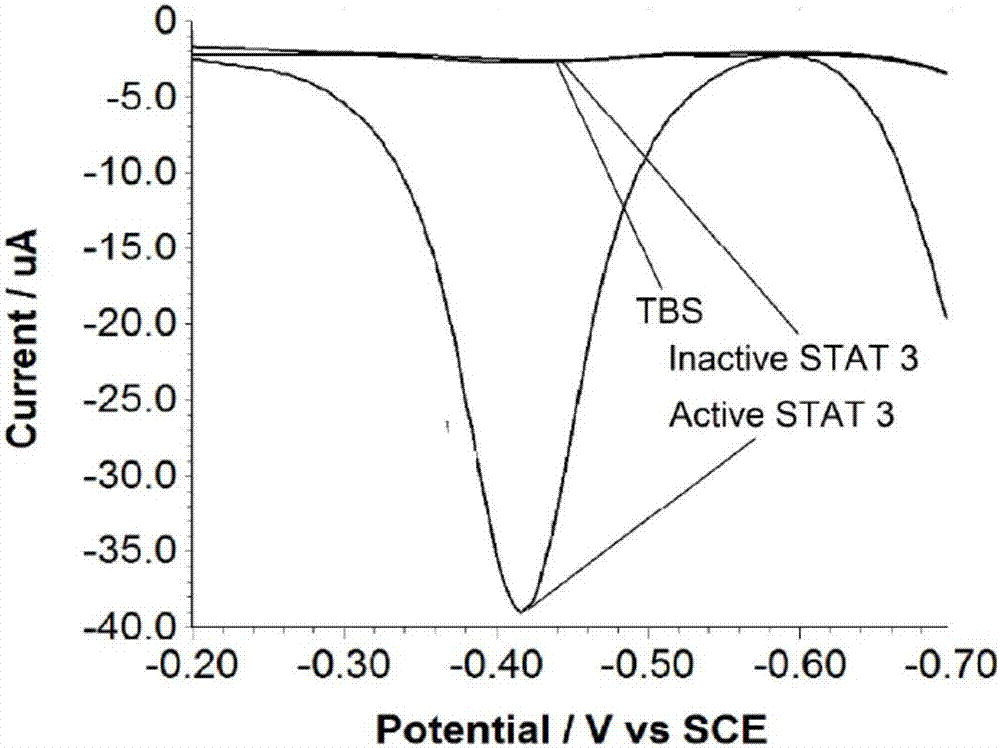
[0062] The gold electrode modified with the double-stranded DNA sequence of the capture probe was reacted with 100nM activated STAT 3 protein, 100nM non-activated STAT 3 protein, and 10mM TBS buffer (pH 7.4) respectively to investigate the effect of the present invention on the activated STAT 3 protein specificity.
[0063] Figure 4 In this study, when the functionalized gold electrode reacted with the activated STAT 3 protein through a series of experimental steps and finally introduced the graphene catalytic beacon, the catalyzed aniline oxidation reaction produced a large amount of 2,3- Diaminophenazine, resulting in a very pronounced SWV peak at -0.42mV. However, when the activated protein was replaced with the same concentration of non-activated STAT 3 protein or TBS buffer was used as a blank control to react with the electrode, since there was no protein binding on the electrode surface, all DNA was degraded, and ultimately no available Zr 4+ The identified phosphory...
Embodiment 3
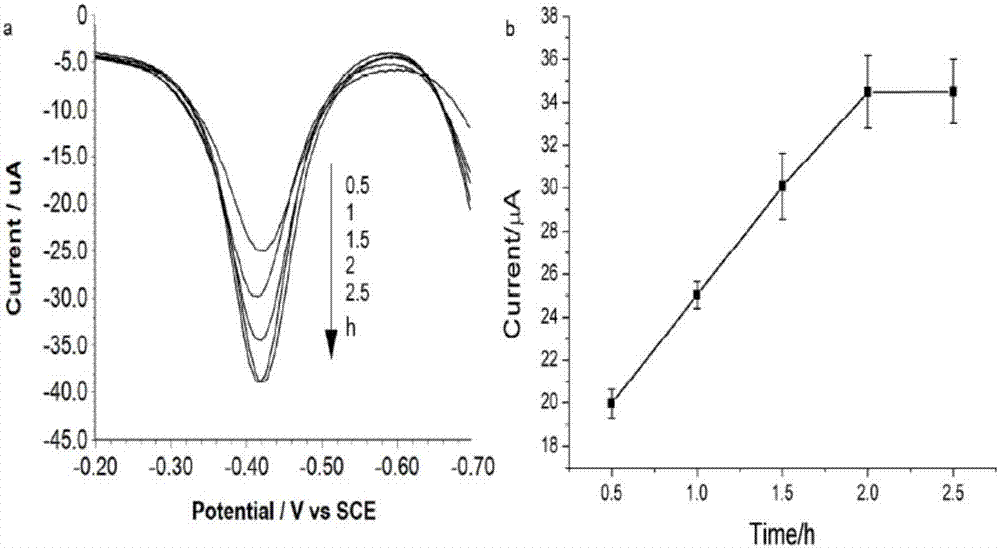
[0065] Optimization of protein incubation time with capture probes. Considering that being able to capture the activated STAT 3 protein from the liquid phase and successfully immobilize it to the electrode interface is an important basis for the whole process, so this example sets the incubation time between the STAT 3 protein and the DNA capture probe on the electrode optimized ( image 3 ). The experimental results show that the peak current value of SWV reaches saturation at 120 minutes, which is enough to ensure that the activated STAT 3 can be recognized and bound by the DNA sequence of the capture probe, thereby being immobilized on the surface of the gold electrode. The incubation time for one step was chosen to be 120 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com